anti-Selective Asymmetric Michael Reactions of Aldehydes and Nitroolefins Catalyzed by a Primary Amine/Thiourea†
Hisatoshi Uehara Dr.
Departments of Chemistry and Molecular Biology and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2583 http://www.scripps.edu/mb/barbas
Search for more papers by this authorCarlos F. Barbas III Prof. Dr.
Departments of Chemistry and Molecular Biology and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2583 http://www.scripps.edu/mb/barbas
Search for more papers by this authorHisatoshi Uehara Dr.
Departments of Chemistry and Molecular Biology and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2583 http://www.scripps.edu/mb/barbas
Search for more papers by this authorCarlos F. Barbas III Prof. Dr.
Departments of Chemistry and Molecular Biology and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA), Fax: (+1) 858-784-2583 http://www.scripps.edu/mb/barbas
Search for more papers by this authorWe thank the Skaggs Institute for Chemical Biology for funding.
Graphical Abstract
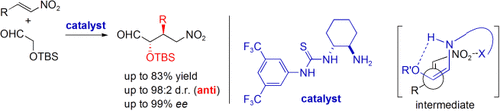
It′s finally here: Highly anti-selective Michael reactions of a functionalized aldehyde with nitroolefins have been realized using a primary amine/thiourea catalyst (see scheme; TBS=tert-butyldimethylsilyl). The reaction relies on a conformational strategy based on directing the formation of a Z-configured enamine intermediate.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200905313_sm_miscellaneous_information.pdf3.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on asymmetric organocatalysis, see:
- 1aP. Melchiorre, M. Marigo, A. Carlone, G. Bartoli, Angew. Chem. 2008, 120, 6232–6265;
10.1002/ange.200705523 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6138–6171;
- 1bC. F. Barbas III, Angew. Chem. 2008, 120, 44–50;
10.1002/ange.200702210 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 42–47;
- 1cS. Mukherjee, J. W. Yang, S. Hoffmann, B. List, Chem. Rev. 2007, 107, 5471–5569;
- 1dA. G. Doyle, E. N. Jacobsen, Chem. Rev. 2007, 107, 5713–5743.
- 2
- 2aS. Mitsumori, H. Zhang, P. H.-Y. Cheong, K. N. Houk, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 1040–1041;
- 2bH. Zhang, M. Mifsud, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 9630–9631;
- 2cH. Zhang, S. Mitsumori, N. Utsumi, M. Imai, N. Garcia-Delgado, M. Mifsud, K. Albertshofer, P. H.-Y. Cheong, K. N. Houk, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2008, 130, 875–886.
- 3
- 3aS. S. V. Ramasastry, H. Zhang, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2007, 129, 288–289;
- 3bS. S. V. Ramasastry, K. Albertshofer, N. Utsumi, F. Tanaka, C. F. Barbas III, Angew. Chem. 2007, 119, 5668–5671;
10.1002/ange.200701269 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5572–5575;
- 3cN. Utsumi, M. Imai, F. Tanaka, S. S. V. Ramasastry, C. F. Barbas III, Org. Lett. 2007, 9, 3445–3448;
- 3dS. S. V. Ramasastry, K. Albertshofer, N. Utsumi, C. F. Barbas III, Org. Lett. 2008, 10, 1621–1624;
- 3eH. Zhang, S. S. V. Ramasastry, F. Tanaka, C. F. Barbas III, Adv. Synth. Catal. 2008, 350, 791–796.
- 4For other selected studies concerning the organocatalytic anti-Mannich reactions, see:
- 4aT. Kano, Y. Yamaguchi, O. Tokuda, K. Maruoka, J. Am. Chem. Soc. 2005, 127, 16408–16409;
- 4bJ. Franzén, M. Marigo, D. Fielenbach, T. C. Wabnitz, A. Kjærsgaard, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 18296–18304;
- 4cT. Kano, Y. Hato, K. Maruoka, Tetrahedron Lett. 2006, 47, 8467–8469;
- 4dP. Dziedzic, A. Córdova, Tetrahedron: Asymmetry 2007, 18, 1033–1037;
- 4eQ.-X. Guo, H. Liu, C. Guo, S.-W. Luo, Y. Gu, L.-Z. Gong, J. Am. Chem. Soc. 2007, 129, 3790–3791;
- 4fM. Pouliquen, J. Blanchet, M. C. Lasne, J. Rouden, Org. Lett. 2008, 10, 1029–1032;
- 4gC. Gianelli, L. Sambri, A. Carlone, G. Bartoli, P. Melchiorre, Angew. Chem. 2008, 120, 8828–8830;
10.1002/ange.200803819 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8700–8702;
- 4hT. Kano, Y. Yamaguchi, K. Maruoka, Angew. Chem. 2009, 121, 1870–1872;
10.1002/ange.200805628 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1838–1840.
- 5For other selected studies concerning organocatalytic syn-aldol reactions, see:
- 5aT. Kano, Y. Yamaguchi, Y. Tanaka, K. Maruoka, Angew. Chem. 2007, 119, 1768–1770;
10.1002/ange.200604640 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1738–1740;
- 5bS. Luo, H. Xu, J. Li, L. Zhang, J.-P. Cheng, J. Am. Chem. Soc. 2007, 129, 3074–3075;
- 5cM. Markert, M. Mulzer, B. Schetter, R. Mahrwald, J. Am. Chem. Soc. 2007, 129, 7258–7259;
- 5dS. Luo, H. Xu, L. Chen, J.-P. Cheng, Org. Lett. 2008, 10, 1775–1778;
- 5eN. Palyam, M. Majewski, J. Org. Chem. 2009, 74, 4390–4392.
- 6For recent reviews on asymmetric organocatalytic Michael reactions, see:
- 6aS. B. Tsogoeva, Eur. J. Org. Chem. 2007, 1701–1716;
- 6bJ. L. Vicario, D. Badía, L. Carrillo, Synthesis 2007, 2065–2092;
- 6cS. Sulzer-Mossé, A. Alexakis, Chem. Commun. 2007, 3123–3135.
- 7For selected examples of syn-selective organocatalytic Michael reactions of aldehydes and nitroolefins, see:
- 7aJ. M. Betancort, C. F. Barbas III, Org. Lett. 2001, 3, 3737–3740;
- 7bA. Alexakis, O. Andrey, Org. Lett. 2002, 4, 3611–3614;
- 7cJ. M. Betancort, K. Sakthivel, R. Thayumanavan, F. Tanaka, C. F. Barbas III, Synthesis 2004, 1509–1521;
- 7dW. Wang, J. Wang, H. Li, Angew. Chem. 2005, 117, 1393–1395; Angew. Chem. Int. Ed. 2005, 44, 1369–1371;
- 7eY. Hayashi, H. Gotoh, T. Hayashi, M. Shoji, Angew. Chem. 2005, 117, 4284–4287; Angew. Chem. Int. Ed. 2005, 44, 4212–4215;
- 7fN. Mase, K. Watanabe, H. Yoda, K. Takabe, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 4966–4967;
- 7gS. Zhu, S. Yu, D. Ma, Angew. Chem. 2008, 120, 555–558; Angew. Chem. Int. Ed. 2008, 47, 545–548;
- 7hM. Wiesner, J. D. Revell, H. Wennemers, Angew. Chem. 2008, 120, 1897–1900;
10.1002/ange.200704972 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1871–1874.
- 8Anti-selective organocatalytic Michael reactions of ketones and nitroolefins were known, see:
- 8aO. Andrey, A. Alexakis, G. Bernardinelli, Org. Lett. 2003, 5, 2559–2561;
- 8bO. Andrey, A. Alexakis, A. Tomassini, G. Bernardinelli, Adv. Synth. Catal. 2004, 346, 1147–1168;
- 8cS. B. Tsogoeva, S. Wei, Chem. Commun. 2006, 1451–1453;
- 8dH. Huang, E. N. Jacobsen, J. Am. Chem. Soc. 2006, 128, 7170–7171;
- 8eY. Xu, W. Zou, H. Sundén, I. Ibrahem, A. Córdova, Adv. Synth. Catal. 2006, 348, 418–424;
- 8fD. Enders, S. Chow, Eur. J. Org. Chem. 2006, 4578–4584;
- 8gT. Mandal, C.-G. Zhao, Angew. Chem. 2008, 120, 7828–7831; Angew. Chem. Int. Ed. 2008, 47, 7714–7717.
- 9For pioneering studies on Michael reactions of preformed enamines and enolates, see:
- 9aD. Seebach, J. Goliński, Helv. Chim. Acta 1981, 64, 1413–1423;
- 9bS. J. Blarer, W. B. Schweizer, D. Seebach, Helv. Chim. Acta 1982, 65, 1637–1654;
- 9cR. Haner, T. Laube, D. Seebach, Chimia 1984, 38, 255–257;
- 9dD. Seebach, A. K. Beck, J. Goliński, J. N. Hay, T. Laube, Helv. Chim. Acta 1985, 68, 162–172.
- 10T. Hoffmann, G. Zhong, B. List, D. Shabat, J. Anderson, S. Gramatikova, R. A. Lerner, C. F. Barbas III, J. Am. Chem. Soc. 1998, 120, 2768–2779.
- 11There is one example proposing Z-enamine formation from benzyloxyacetaldehyde with primary amino acids in self-aldol reactions. Diastereoselectivity, however, was low (anti/syn 2:1) and surprisingly anti favored as in the typical proline-catalyzed aldol reaction. A. Córdova, I. Ibrahem, J. Casas, H. Sundén, M. Engqvist, E. Reyes, Chem. Eur. J. 2005, 11, 4772–4784.
- 12For primary amine catalyzed Michael reactions of aldehydes and nitroolefins, see:
- 12aM. P. Lalonde, Y. Chen, E. N. Jacobsen, Angew. Chem. 2006, 118, 6514–6518;
10.1002/ange.200602221 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6366–6370;
- 12bY. Xu, W. Zou, H. Sundén, I. Ibrahem, A. Córdova, Adv. Synth. Catal. 2006, 348, 418–424;
- 12cS. H. McCooey, S. J. Connon, Org. Lett. 2007, 9, 599–602;
- 12dA. Sato, M. Yoshida, S. Hara, Chem. Commun. 2008, 6242–6244;
- 12eX.-J. Zhang, S.-P. Liu, X.-M. Li, M. Yan, A. S. C. Chan, Chem. Commun. 2009, 833–835;
- 12fX.-J. Zhang, S.-P. Liu, J.-H. Lao, G.-J. Du, M. Yan, A. S. C. Chan, Tetrahedron: Asymmetry 2009, 20, 1451–1458.
- 13The absolute and relative configuration were determined by converting the product 3 into a known compound and comparing the 1H NMR data and optical rotation data: M. Ayerbe, I. Morao, A. Arrieta, A. Linden, F. P. Cossío, Tetrahedron Lett. 1996, 37, 3055–3058; also see the Supporting Information. TBAF=tetra-n-butylammonium fluoride, CSA=(±)-camphorsulfonic acid.

- 14
- 14aF. Xue, S. Zhang, W. Duan, W. Wang, Adv. Synth. Catal. 2008, 350, 2194–2198;
- 14bB. Mohar, A. Valleix, J.-R. Desmurs, M. Felemez, A. Wagner, C. Mioskowski, Chem. Commun. 2001, 2572–2573.
- 15K. Mei, M. Jin, S. Zhang, P. Li, W. Liu, X. Chen, F. Xue, W. Duan, W. Wang, Org. Lett. 2009, 11, 2864–2867; also see: reference [12f], and references therein.
- 16It is noteworthy that Jacobsen’s group reported syn diastereoselectivity when they used 2-(4-methoxybenzyloxy)propanal, see reference [12a].
- 17For the catalytic mechanism of L-Phe-OLi (6) and O-tBu-L-Thr-OLi (7), we propose a transition-state model with coordination of lithium to nitroolefins as an alternative model to that proposed by Yoshida and co-workers in reference [12d]: see the Supporting Information.
- 18For a related report, including computational analysis, see: D. A. Yalalov, S. B. Tsogoeva, S. Schmatz, Adv. Synth. Catal. 2006, 348, 826–832.
- 19Mono-Boc-protected cyclohexanediamine was used in a Michael reaction of isobutyraldehyde and β-nitrostyrene, see: reference [12f].
- 20O. Andrey, A. Alexakis, A. Tomassini, G. Bernardinelli, Adv. Synth. Catal. 2004, 346, 1147–1168.