Catalytic Asymmetric 6π Electrocyclization: Enantioselective Synthesis of Functionalized Indolines†
Eleanor E. Maciver
Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA (UK) http://msmith.chem.ox.ac.uk
Search for more papers by this authorSam Thompson Dr.
Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA (UK) http://msmith.chem.ox.ac.uk
Search for more papers by this authorMartin D. Smith Dr.
Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA (UK) http://msmith.chem.ox.ac.uk
Search for more papers by this authorEleanor E. Maciver
Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA (UK) http://msmith.chem.ox.ac.uk
Search for more papers by this authorSam Thompson Dr.
Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA (UK) http://msmith.chem.ox.ac.uk
Search for more papers by this authorMartin D. Smith Dr.
Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA (UK) http://msmith.chem.ox.ac.uk
Search for more papers by this authorWe acknowledge funding from the Royal Society (MDS), GSK and the EPSRC (E.E.M. and S.T.), the University of Oxford John Fell Fund and AstraZeneca. We thank Dr. John Ward (GSK) and Dr. Andrew Cridland (GSK) for useful discussions, Dr. Amber Thompson for assistance with X-ray crystallography, and Prof. Ian Fleming FRS for insightful comments.
Graphical Abstract
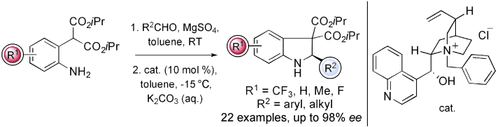
How to close a ring: An approach to catalytic asymmetric 6π electrocyclization leads to a highly enantioselective process that was used in the synthesis of chiral indolines (see scheme). Treatment of N-aryl imines under phase transfer conditions in the presence of N-benzyl cinchonidinium chloride generates a delocalized 2-aza-pentadienyl anion system that cyclizes in up to 99 % yield and 98 % ee.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200905169_sm_miscellaneous_information.pdf18.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. B. Woodward, R. Hoffmann, The Conservation of Orbital Symmetry, Verlag Chemie, Weinheim, 1970.
- 2aR. B. Woodward, Pure Appl. Chem. 1968, 17, 519;
- 2bA. R. Todd, J. W. Cornforth, Biogr. Mem. Fellows R. Soc. 1981, 27, 628.
- 3C. M. Beaudry, J. M. Malerich, D. Trauner, Chem. Rev. 2005, 105, 4757.
- 4
- 4aG. X. Liang, S. N. Gradl, D. Trauner, Org. Lett. 2003, 5, 4931;
- 4bV. K. Aggarwal, A. J. Belfield, Org. Lett. 2003, 5, 5075;
- 4cJ. Nie, H.-W. Zhu, H.-F. Cui, M.-Q. Hua, J.-A. Ma, Org. Lett. 2007, 9, 3053;
- 4dM. Rueping, W. Ieawsuwan, A. P. Antonchick, B. J. Nachtsheim, Angew. Chem. 2007, 119, 2143;
10.1002/ange.200604809 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2097;
- 4eH.-F. Cui, K.-Y. Dong, G.-W. Zhang, L. Wang, J.-A. Ma, Chem. Commun. 2007, 2284;
- 4fI. Walz, A. Togni, Chem. Commun. 2008, 4315.
- 5For catalytic Nazarov cyclizations by asymmetric protonation see:
- 5aG. X. Liang, D. Trauner, J. Am. Chem. Soc. 2004, 126, 9544;
- 5bM. Rueping, W. Ieawsuwan, Adv. Synth. Catal. 2009, 351, 78.
- 6For a hexatriene electrocyclization catalyzed by a Lewis acid see: L. M. Bishop, J. E. Barbarow, R. G. Bergman, D. Trauner, Angew. Chem. 2008, 120, 8220; Angew. Chem. Int. Ed. 2008, 47, 8100.
- 7C. W. Shoppee, G. N. Henderson, J. Chem. Soc. Chem. Commun. 1974, 561.
- 8J. C. Hodges, W. Wang, F. Riley, J. Org. Chem. 2004, 69, 2504.
- 9
- 9aN. A. Magomedov, P. L. Ruggiero, Y. C. Tang, J. Am. Chem. Soc. 2004, 126, 1624;
- 9bN. A. Magomedov, P. L. Ruggiero, Y. C. Tang, Org. Lett. 2004, 6, 3373;
- 9cT. Q. Yu, Y. Fu, L. Liu, Q. X. Guo, J. Org. Chem. 2006, 71, 6157;
- 9dT. J. Greshock, R. L. Funk, J. Am. Chem. Soc. 2006, 128, 4946.
- 10
- 10aD. H. Hunter, R. P. Steiner, Can. J. Chem. 1975, 53, 355;
- 10bS. Klötgen, R. Frölich, E. U. Würthwein, Tetrahedron 1996, 52, 14801;
- 10cK. Gerdes, P. Sagar, R. Frölich, B. Wibbeling, E. U. Würthwein, Eur. J. Org. Chem. 2004, 3465.
- 11
- 11aW. N. Speckamp, S. J. Veenstra, J. Dijkink, R. Fortgens, J. Am. Chem. Soc. 1981, 103, 4643;
- 11bS. J. Veenstra, W. N. Speckamp, J. Am. Chem. Soc. 1981, 103, 4645;
- 11cS. J. Veenstra, W. N. Speckamp, J. Chem. Soc. Chem. Commun. 1982, 369;
- 11dJ. Dijkink, J. N. Zonjee, B. S. de Jong, W. N. Speckamp, Heterocycles 1983, 20, 1255;
- 11eW. N. Speckamp, Heterocycles 1984, 21, 211;
- 11fS. J. Veenstra, H. P. Fortgens, R. J. Vijn, B. S. de Jong, W. N. Speckamp, Tetrahedron 1987, 43, 1147.
- 12
- 12aR. Huisgen, Angew. Chem. 1980, 92, 979; Angew. Chem. Int. Ed. Engl. 1980, 19, 947;
- 12bE. C. Taylor, I. Turchi, Chem. Rev. 1979, 79, 181;
- 12cR. Grigg, H. Q. N. Gunaratne, Tetrahedron Lett. 1983, 24, 1201;
- 12dR. Grigg, H. Q. N. Gunaratne, J. Kemp, J. Chem. Soc. Perkin Trans. 1 1984, 31;
- 12eR. Grigg, H. Q. N. Gunaratne, D. Henderson, V. Sridharan, Tetrahedron 1990, 46, 1599;
- 12fC. B. Kanner, U. K. Pandit, Tetrahedron 1981, 37, 3519.
- 13For an asymmetric [1,5]-electrocyclic reaction see: R. J. Vijn, W. N. Speckamp, B. S. de Jong, H. Hiemstra, Angew. Chem. 1984, 96, 165; Angew. Chem. Int. Ed. Engl. 1984, 23, 165.
- 14For a dearomatizing 6π electrocyclic process see: J. Clayden, S. Purewal, M. Helliwell, S. J. Mantell, Angew. Chem. 2002, 114, 1091; Angew. Chem. Int. Ed. 2002, 41, 1049.
- 15
- 15aW. Kirmse, N. G. Rondan, K. N. Houk, J. Am. Chem. Soc. 1984, 106, 7989;
- 15bN. G. Rondan, K. N. Houk, J. Am. Chem. Soc. 1985, 107, 2099.
- 16For reviews of asymmetric phase transfer catalysis see:
- 16aM. J. O’Donnell in Catalytic Asymmetric Synthesis, 2nd ed. (Ed.: ), Wiley-VCH, Weinheim, 2000, p. 727;
10.1002/0471721506.ch23 Google Scholar
- 16bT. Ooi, K. Maruoka, Angew. Chem. 2007, 119, 4300;
10.1002/ange.200601737 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4222.
- 17For other asymmetric transformations influenced by a chiral counterion, see:
- 17aM. S. Taylor, E. N. Jacobsen, Angew. Chem. 2006, 118, 1550; Angew. Chem. Int. Ed. 2006, 45, 1520;
- 17bS. Mayer, B. List, Angew. Chem. 2006, 118, 4299; Angew. Chem. Int. Ed. 2006, 45, 4193;
- 17cT. Akiyama, J. Itoh, K. Fuchibe, Adv. Synth. Catal. 2006, 348, 999;
- 17dM. Rueping, E. Sugiono, C. Azap, T. Theissmann, M. Bolte, Org. Lett. 2005, 7, 3781;
- 17eG. L. Hamilton, E. J. Kang, M. Mba, F. D. Toste, Science 2007, 317, 496.
- 18F. G. Goetz, J. A. Hirsch, R. L. Augustine, J. Org. Chem. 1983, 48, 2468.
- 19See Supporting Information for further details.
- 20For asymmetric Mannich reactions that proceed under phase-transfer catalysis, see:
- 20aT. Ooi, M. Kameda, J. Fujii, K. Maruoka, Org. Lett. 2004, 6, 2397;
- 20bT. B. Poulsen, C. Alemparte, S. Saaby, M. Bella, K. A. Jørgensen, Angew. Chem. 2005, 117, 2956;
10.1002/ange.200500144 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2896;
- 20cA. Okada, T. Shibuguchi, T. Oshima, H. Masu, K. Yamaguchi, M. Shibasaki, Angew. Chem. 2005, 117, 4640; Angew. Chem. Int. Ed. 2005, 44, 4564;
- 20dF. Fini, L. Bernardi, R. P. Herrera, D. Pettersen, A. Ricci, V. Sgarzani, Adv. Synth. Catal. 2006, 348, 2043;
- 20eB. Niess, K. A. Jørgensen, Chem. Commun. 2007, 1620;
- 20fT. Shibuguchi, H. Mihara, A. Kuramochi, T. Ohshima, M. Shibasaki, Chem. Asian J. 2007, 2, 794.
- 21
- 21aJ. E. Baldwin, J. Chem. Soc. Chem. Commun. 1976, 734;
- 21bJ. E. Baldwin, J. Org. Chem. 1977, 42, 3846.
- 22For an example of an apparent 5-endo-trig process involving an imine, see: R. Grigg, J. Kemp, J. Malone, A. Tangthongkum, J. Chem. Soc. Chem. Commun. 1980, 648.
- 23Hydrogen bonding to the imine could mediate a facially selective intramolecular Mannich reaction, and could account for the apparent importance of the hydroxy group for high enantioselectivity. Addition of hydrogen bonding catalysts (such as chiral thioureas or free cinchonidine/cinchonine alkaloids) lowered the rate of reaction and produced racemic material.
- 24E. J. Corey, F. Xu, M. C. Noe, J. Am. Chem. Soc. 1997, 119, 12414.
- 25The role of the hydroxy group in cinchona alkaloid-derived phase-transfer catalysts has been discussed at length: M. J. O’Donnell, S. Wu, J. C. Huffman, Tetrahedron 1994, 50, 4507.