A Calix[4]arene 3d/4f Magnetic Cooler†
Georgios Karotsis
School of Chemistry, The University of Edinburgh, West Mains Road, Edinburgh, EH9 3JJ (UK), Fax: (+44) 131-650-6453
Search for more papers by this authorMarco Evangelisti Dr.
Instituto de Ciencia de Materiales de Aragón, CSIC - Universidad de Zaragoza, Departamento de Fisica de la Materia Condensada, 50009 Zaragoza (Spain)
Search for more papers by this authorScott J. Dalgarno Dr.
School of Engineering and Physical Sciences, Heriot-Watt University, Riccarton, Edinburgh, EH14 4AS (UK), Fax: (+44) 131-451-3180
Search for more papers by this authorEuan K. Brechin Dr.
School of Chemistry, The University of Edinburgh, West Mains Road, Edinburgh, EH9 3JJ (UK), Fax: (+44) 131-650-6453
Search for more papers by this authorGeorgios Karotsis
School of Chemistry, The University of Edinburgh, West Mains Road, Edinburgh, EH9 3JJ (UK), Fax: (+44) 131-650-6453
Search for more papers by this authorMarco Evangelisti Dr.
Instituto de Ciencia de Materiales de Aragón, CSIC - Universidad de Zaragoza, Departamento de Fisica de la Materia Condensada, 50009 Zaragoza (Spain)
Search for more papers by this authorScott J. Dalgarno Dr.
School of Engineering and Physical Sciences, Heriot-Watt University, Riccarton, Edinburgh, EH14 4AS (UK), Fax: (+44) 131-451-3180
Search for more papers by this authorEuan K. Brechin Dr.
School of Chemistry, The University of Edinburgh, West Mains Road, Edinburgh, EH9 3JJ (UK), Fax: (+44) 131-650-6453
Search for more papers by this authorWe thank the EPSRC and Heriot-Watt University for financial support of this work. M.E. acknowledges grants MAT2009-13977-C03 and CSD2007-00010, and funding from the RyC program, all from the Spanish Ministry for Science and Innovation.
Graphical Abstract
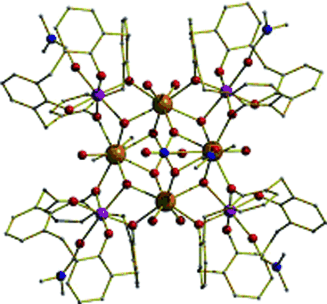
Chilling out: The first 3d/4f cluster based on calix[4]arenes (see picture; purple Mn, brown Gd, red O, blue N) has a high magnetic isotropy and a large number of molecular spin states that are populated even at low temperatures, whereas its ferromagnetic limit is approached only at high applied fields. These results enable the complex to be an excellent magnetic refrigerant for low-temperature applications.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200905012_sm_miscellaneous_information.pdf660.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. E. P. Winpenny, J. Chem. Soc. Dalton Trans. 2002, 1–10.
- 2D. Gatteschi, R. Sessoli, J. Villain, Molecular Nanomagnets, Oxford University Press, 2006, and references therein.
- 3M. Affronte, F. Troiani, A. Ghirri, S. Carretta, P. Santini, V. Corradini, R. Schuecker, C. Muryn, G. Timco, R. E. Winpenny, Dalton Trans. 2006, 2810–2817.
- 4L. Bogani, W. Wernsdorfer, Nat. Mater. 2008, 7, 179–186.
- 5
- 5aJ. R. Friedman, M. P. Sarachik, J. Tejada, R. Ziolo, Phys. Rev. Lett. 1996, 76, 3830;
- 5bL. Thomas, F. Lionti, R. Ballou, D. Gatteschi, R. Sessoli, B. Barbara, Nature 1996, 383, 145–147;
- 5cA. Garg, Europhys. Lett. 1993, 22, 205;
- 5dW. Wernsdorfer, R. Sessoli, Science 1999, 284, 133–135.
- 6
- 6aR. E. P. Winpenny in Comprehensive Coordination Chemistry II, Vol. 7 (Ed.: ), Elsevier, Amsterdam, 2004, pp. 125–175;
10.1016/B0-08-043748-6/06199-5 Google Scholar
- 6bA. J. Tasiopoulos, S. P. Perlepes, Dalton Trans. 2008, 5537–5555.
- 7G. Aromí, E. K. Brechin, Struct. Bonding (Berlin) 2006, 122, 1–69.
- 8
- 8aC. Ritchie, A. Ferguson, H. Nojiri, H. N. Miras, Y.-F. Song, D.-L. Long, E. Burkholder, M. Murrie, P. Kögerler, E. K. Brechin, L. Cronin, Angew. Chem. 2008, 120, 5691–5694;
10.1002/ange.200801281 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5609–5612;
- 8bM. A. AlDamen, J. M. Clemente-Juan, E. Coronado, C. Martí-Castaldo, A. Gaita-Ariño, J. Am. Chem. Soc. 2008, 130, 8874–8875;
- 8cJ.-D. Compain, P. Mialane, A. Dolbecq, I. Martyr Mbomekallé, J. Marrot, F. Sécheresse, E. Rivière, G. Rogez, W. Wernsdorfer, Angew. Chem. 2009, 121, 3123–3127;
10.1002/ange.200900117 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3077–3081.
- 9For example, see: L. R. MacGillivray, J. L. Atwood, Nature 1997, 389, 469–472;
O. Ugono, K. T. Holman, Chem. Commun. 2006, 2144–2146;
T. Gerkensmeier, W. Iwanek, C. Agena, R. Froehlich, S. Kotila, C. Nather, J. Mattay, Eur. J. Org. Chem. 1999, 2257–2262;
10.1002/(SICI)1099-0690(199909)1999:9<2257::AID-EJOC2257>3.0.CO;2-H CAS Web of Science® Google ScholarE. S. Barrett, T. J. Dale, J. Rebek, Jr., J. Am. Chem. Soc. 2007, 129, 3818–3819; S. J. Dalgarno, S. A. Tucker, D. B. Bassil, J. L. Atwood, Science 2005, 309, 2037–2039.
- 10For example, see: G. W. Orr, L. J. Barbour, J. L. Atwood, Science 1999, 285, 1049–1052;
J. L. Atwood, L. J. Barbour, S. J. Dalgarno, M. J. Hardie, C. L. Raston, H. R. Webb, J. Am. Chem. Soc. 2004, 126, 13170–13171;
R. M. McKinlay, G. W. V. Cave, J. L. Atwood, Proc. Natl. Acad. Sci. USA 2005, 102, 5944–5948;
T. K. Ronson, J. Fisher, L. P. Harding, M. J. Hardie, Angew. Chem. 2007, 119, 9244–9246;
Angew. Chem. Int. Ed. 2007, 46, 9086–9088;
N. P. Power, S. J. Dalgarno, J. L. Atwood, Angew. Chem. 2007, 119, 8755–8758;
10.1002/ange.200702941 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8601–8604; S. J. Dalgarno, N. P. Power, J. E. Warren, J. L. Atwood, Chem. Commun. 2008, 1539–1541; O. Ugono, J. P. Moran, K. T. Holman, Chem. Commun. 2008, 1404–1406.
- 11C. Aronica, G. Chastanet, E. Zueva, S. A. Borshch, J. M. Clemente-Juan, D. Luneau, J. Am. Chem. Soc. 2008, 130, 2365–2371.
- 12For a recent review on transition-metal and lanthanide cluster complexes formed with thiacalix[n]arenes, see: T. Kajiwara, N. Iki, M. Yamashita, Coord. Chem. Rev. Coord. Chem. Rev.2007, 251, 1734–1746, and references therein.
- 13Y. Bi, X.-T. Wang, W. Liao, X. Wang, X. Wang, H. Zhang, S. Gao, J. Am. Chem. Soc. 2009, 131, 11650–11651.
- 14G. Karotsis, S. J. Teat, W. Wernsdorfer, S. Piligkos, S. J. Dalgarno, E. K. Brechin, Angew. Chem. 2009, 131, 11650–11651; Angew. Chem. Int. Ed. 2009, 48, 8151–8379.
- 15For a paper highlighting recent advances in 3d/4f cluster chemistry, see: A. M. Ako, V. Mereacre, R. Clérac, I. J. Hewitt, Y. Lan, G. Buth, C. E. Anson, A. K. Powell, Inorg. Chem. 2009, 48, 6713–6723, and references therein.
- 16
- 16aM. Manoli, A. Collins, S. Parsons, A. Candini, M. Evangelisti, E. K. Brechin, J. Am. Chem. Soc. 2008, 130, 11129–11139;
- 16bM. Evangelisti, A. Candini, M. Affronte, E. Pasca, L. J. de Jongh, R. T. W. Scott, E. K. Brechin, Phys. Rev. B 2009, 79, 104414.
- 17
- 17aM. Evangelisti, A. Candini, A. Ghirri, M. Affronte, E. K. Brechin, E. J. L. McInnes, Appl. Phys. Lett. 2005, 87, 072504;
- 17bM. Evangelisti, F. Luis, L. J. de Jongh, M. Affronte, J. Mater. Chem. 2006, 16, 2534–2549;
- 17cR. Shaw, R. H. Laye, L. F. Jones, D. M. Low, C. Talbot-Eeckelaers, Q. Wei, C. J. Milios, S. Teat, M. Helliwell, J. Raftery, M. Evangelisti, M. Affronte, D. Collison, E. K. Brechin, E. J. L. McInnes, Inorg. Chem. 2007, 46, 4968–4978;
- 17dM. Manoli, R. D. L. Johnstone, S. Parsons, M. Murrie, M. Affronte, M. Evangelisti, E. K. Brechin, Angew. Chem. 2007, 119, 4540–4544;
10.1002/ange.200701027 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4456–4460.
- 18
- 18aC. Zimm, A. Jastrab, A. Sternberg, V. Pecharsky, K. Gschneidner, Jr., M. Osborne, I. Anderson, Adv. Cryog. Eng. 1998, 43, 1759–1766;
- 18bV. K. Pecharsky, K. A. Gschneidner, Jr., J. Magn. Magn. Mater. 1999, 200, 44–56;
- 18cK. A. Gschneidner, Jr., A. O. Pecharsky, V. K. Pecharsky in Cryocoolers II (Ed.: ), Kluwer Academic/Plenum Press, New York, 2001, p. 433.
- 19A. L. Spek, Acta Crystallogr. Sect. A 1990, 46, C 34.