Intramolecular Alkynylcyclopropanation of Olefins Catalyzed by Bi(OTf)3: Stereoselective Synthesis of 1-Alkynyl-3-azabicyclo[3.1.0]hexanes†
Kimihiro Komeyama Dr.
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorNatsuko Saigo
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorMotoyoshi Miyagi
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorKen Takaki Prof. Dr.
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorKimihiro Komeyama Dr.
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorNatsuko Saigo
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorMotoyoshi Miyagi
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorKen Takaki Prof. Dr.
Department of Chemistry and Chemical Engineering, Graduate School of Engineering, Hiroshima University, Kagamiyama, Higashi-Hiroshima 739-8527 (Japan), Fax: (+81) 82-424-5494
Search for more papers by this authorWe thank H. Fukuoka for X-ray analysis. This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT). K.K. acknowledges financial support from the Electric Technology Research Foundation of Chugoku.
Graphical Abstract
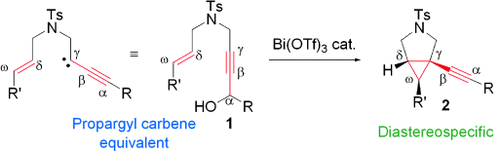
The postman always rings twice: 1-Alkynyl-3-azabicyclo[3.1.0]hexanes 2 were obtained in good to excellent yields from the stereoselective Bi(OTf)3-catalyzed dehydrative alkynylcyclopropanation of azaenynols 1, in which the propargyl alcohol motif of 1 acted as a propargyl carbene synthetic equivalent.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200904610_sm_miscellaneous_information.pdf376.9 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Zhang, J. Sun, S. A. Kozmin, Adv. Synth. Catal. 2006, 348, 2271;
- 1bV. Michelet, P. Y. Toullec, J. P. Genêt, Angew. Chem. 2008, 120, 4338;
10.1002/ange.200701589 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4268;
- 1cE. Jiménez-Núñez, A. M. Echavarren, Chem. Rev. 2008, 108, 3326.
- 2For examples using iron catalysis, see:
- 2aK. Komeyama, T. Morimoto, K. Takaki, Angew. Chem. 2006, 118, 3004;
10.1002/ange.200503789 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2938;
- 2bK. Komeyama, T. Morimoto, K. Takaki, Tetrahedron Lett. 2007, 48, 3259;
- 2cK. Komeyama, Y. Mieno, S. Yukawa, K. Takaki, Chem. Lett. 2007, 36, 752;
- 2dK. Komeyama, R. Igawa, T. Morimoto, K. Takaki, Chem. Lett. 2009, 38, 724. With Bi catalyst:
- 2eK. Komeyama, K. Takahashi, K. Takaki, Chem. Lett. 2008, 37, 602;
- 2fK. Komeyama, M. Miyagi, K. Takaki, Heteroat. Chem. 2008, 19, 644;
- 2gK. Komeyama, K. Takahashi, K. Takaki, Org. Lett. 2008, 10, 5119;
- 2hK. Komeyama, M. Miyagi, K. Takaki, Chem. Lett. 2009, 38, 224;
- 2iK. Komeyama, R. Igawa, T. Morimoto, K. Takaki, Chem. Lett. 2009, 38, 724.
- 3
- 3aY. Fujimoto, F. Irreverre, J. M. Karle, I. L. Karle, B. J. Witkop, J. Am. Chem. Soc. 1971, 93, 3471;
- 3bS. Gomi, D. Ikeda, H. Nakamura, H. Naganawa, F. Yamashita, K. Hotta, S. Kondo, Y. Okami, H. Umezawa, Y. Iitaka, J. Antibiot. 1984, 37, 1491;
- 3cD. L. Boger, D. S. Johnson, Proc. Natl. Acad. Sci. USA 1995, 92, 3642;
- 3dD. L. Boger, C. W. Boyce, R. M. Garbaccio, J. A. Goldberg, Chem. Rev. 1997, 97, 787.
- 4For examples using titanium reagents, see:
- 4aS. Okamoto, M. Iwakubo, K. Kobayashi, F. Sato, J. Am. Chem. Soc. 1997, 119, 6984;
- 4bA. de Meijere, C. M. Williams, A. Kourdioukov, S. V. Sviridov, V. Chaplinski, M. Kordes, A. I. Savchenko, C. Stratmann, M. Noltemeyer, Chem. Eur. J. 2002, 8, 3789. With sulfur ylide:
10.1002/1521-3765(20020816)8:16<3789::AID-CHEM3789>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 4cM. Es-Sayed, P. Devine, J. E. Burgess, A. de Meijere, A. I. Meyers, J. Chem. Soc. Chem. Commun. 1995, 141. With a radical:
- 4dN. Baldovini, M. P. Bertrand, A. Carrière, R. Nouguier, J. M. Plancher, J. Org. Chem. 1996, 61, 3205.
- 5
- 5aR. Grigg, R. Rasul, J. Redpath, D. Wilson, Tetrahedron Lett. 1996, 37, 46098;
- 5bJ. Böhmer, R. Grigg, J. D. Marchbank, Chem. Commun. 2002, 768.
- 6
- 6aF. Monnier, D. Castillo, S. Dérien, L. Toupet, P. H. Dixneuf, Angew. Chem. 2003, 115, 5632; Angew. Chem. Int. Ed. 2003, 42, 5474;
- 6bF. Monnier, C. V. Bray, D. Castillo, V. Aubert, S. Dérien, P. H. Dixneuf, L. Toupet, A. lenco, C. Mealli, J. Am. Chem. Soc. 2007, 129, 6037.
- 7For the synthesis of five-membered rings, see:
- 7aT. Hudlicky and J. W. Reed in Comprehensive Organic Synthesis, Vol. 5 (Ed.: ), Pergamon, Oxford, 1991, 899;
10.1016/B978-0-08-052349-1.00142-6 Google Scholar
- 7bG. Zuo, J. Louie, Angew. Chem. 2004, 116, 2327; Angew. Chem. Int. Ed. 2004, 43, 2277. For the synthesis of seven-membered rings, see:
- 7cP. A. Wender, H. Takahashi, B. Witulski, J. Am. Chem. Soc. 1995, 117, 4320;
- 7dP. A. Wender, A. J. Dyckman, C. O. Husfeld, D. Kadereit, J. A. Love, H. Rieck, J. Am. Chem. Soc. 1999, 121, 10442;
- 7eB. M. Trost, F. D. Toste, H. Shen, J. Am. Chem. Soc. 2000, 122, 2379. For the synthesis of eight-membered rings, see:
- 7fP. A. Wender, G. G. Gamber, R. D. Hubbard, L. Zhang, J. Am. Chem. Soc. 2002, 124, 2876.
- 8
- 8aE. J. Cho, M. Kim, D. Lee, Org. Lett. 2006, 8, 5413;
- 8bD. Lee. M. Kim, Org. Biomol. Chem. 2007, 5, 3418.
- 9
- 9aM. S. B. Wills, R. L. Danheiser, J. Am. Chem. Soc. 1998, 120, 9378;
- 9bN. Iwasawa, T. Matsuo, M. Iwamoto, T. Ikeno, J. Am. Chem. Soc. 1998, 120, 3903;
- 9cI. Kadota, A. Shibuya, Y. S. Gyoung, Y. Yamamoto, J. Am. Chem. Soc. 1998, 120, 10262;
- 9dK. Ohe, T. Yokoi, K. Miki, F Nishino, S. Uemura, J. Am. Chem. Soc. 2002, 124, 526;
- 9eA. Y. Peng, Y. X. Ding, J. Am. Chem. Soc. 2003, 125, 15006;
- 9fH. Siebeneicher, I. Bytschkov, S. Doye, Angew. Chem. 2003, 115, 3151;
10.1002/ange.200351345 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3042;
- 9gC. García, E. R. Libra, P. J. Carroll, P. J. Walsh, J. Am. Chem. Soc. 2003, 125, 3210;
- 9hB. D. Sherry, F. D. Toste, J. Am. Chem. Soc. 2004, 126, 15978;
- 9iJ. P. Markham, S. T. Staben, F. D. Toste, J. Am. Chem. Soc. 2005, 127, 9708.
- 10
- 10aA. Zampella, M. V. D Auria, L. Minale, C. Debitus, C. Roussakis, J. Am. Chem. Soc. 1996, 118, 11085;
- 10bJ. W. Corbett, S. S. Ko, J. D. Rodgers, L. A. Gearhart, N. A. Magnus, L. T. Bacheler, S. Diamond, S. Jeffrey, R. M. Klabe, B. C. Cordova, S. Garber, K. Logue, G. L. Trainor, P. S. Anderson, S. K. Erickson-Viitanen, J. Med. Chem. 2000, 43, 2019.
- 11
- 11aC. P. Casey, S. Kraft, D. R. Powell, J. Am. Chem. Soc. 2000, 122, 3771;
- 11bJ. Barluenga, M. A. Fernández-Rodríguez, P. García-García, E. Aguilar, I. Merino, Chem. Eur. J. 2006, 12, 303.
- 12
- 12aT. Ishikawa, S. Manabe, T. Aikawa, T. Kudo, S. Saito, Org. Lett. 2004, 6, 2361;
- 12bL. F. Yao, M. Shi, Org. Lett. 2007, 9, 5187.
- 13
- 13aM. Méndez, M. P. Muñoz, A. M. Echavarren, J. Am. Chem. Soc. 2000, 122, 11549;
- 13bM. R. Luzung, J. P. Markham, F. D. Toste, J. Am. Chem. Soc. 2004, 126, 10858;
- 13cK. G. Ji, X. Z. Shu, S. C. Zhao, H. T. Zhu, Y. N. Niu, X. Y. Liu, Y. M. Liang, Org. Lett. 2009, 11, 3206.
- 14C. Fernández-Rivas, M. Méndez, A. M. Echavarren, J. Am. Chem. Soc. 2000, 122, 1221.
- 15
- 15aH. Qin, N. Yamagiwa, S. Matsunaga, M. Shibasaki, Angew. Chem. 2007, 119, 413; Angew. Chem. Int. Ed. 2007, 46, 409.
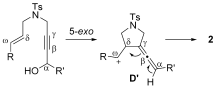
- 16An alternative mechanism that includes the formation of a five-membered intermediate D′, which has a carbocation at the ω-position, is also possible. However, the structure of D′ cannot explain the stereospecificity of the transformation.
- 17A similar mechanism has been proposed in the gold-catalyzed cycloisomerization of 1,5-enynes. See Ref. [13b].