Rotaxane-Based Propeptides: Protection and Enzymatic Release of a Bioactive Pentapeptide†
Anthony Fernandes Dr.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Université de Poitiers, UMR 6514 Laboratoire de Synthèse et Réactivité des Substances Naturelles, 40 Avenue du Recteur Pineau, 86022 Poitiers (France)
These authors contributed equally to this work.
Search for more papers by this authorAurélien Viterisi
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
These authors contributed equally to this work.
Search for more papers by this authorFrédéric Coutrot Dr.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Search for more papers by this authorStéphanie Potok Dr.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Search for more papers by this authorDavid A. Leigh Prof.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Search for more papers by this authorVincent Aucagne Dr.
Centre de Biophysique Moléculaire, UPR 4301 CNRS, Rue Charles Sadron, 45071 Orléans, Cedex 2 (France)
Search for more papers by this authorSébastien Papot Dr.
Université de Poitiers, UMR 6514 Laboratoire de Synthèse et Réactivité des Substances Naturelles, 40 Avenue du Recteur Pineau, 86022 Poitiers (France)
Search for more papers by this authorAnthony Fernandes Dr.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Université de Poitiers, UMR 6514 Laboratoire de Synthèse et Réactivité des Substances Naturelles, 40 Avenue du Recteur Pineau, 86022 Poitiers (France)
These authors contributed equally to this work.
Search for more papers by this authorAurélien Viterisi
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
These authors contributed equally to this work.
Search for more papers by this authorFrédéric Coutrot Dr.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Search for more papers by this authorStéphanie Potok Dr.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Search for more papers by this authorDavid A. Leigh Prof.
School of Chemistry, University of Edinburgh, The King's Buildings, West Mains Road, Edinburgh EH9 3JJ (UK), Fax: (+44) 131-650-6453 http://www.catenane.net
Search for more papers by this authorVincent Aucagne Dr.
Centre de Biophysique Moléculaire, UPR 4301 CNRS, Rue Charles Sadron, 45071 Orléans, Cedex 2 (France)
Search for more papers by this authorSébastien Papot Dr.
Université de Poitiers, UMR 6514 Laboratoire de Synthèse et Réactivité des Substances Naturelles, 40 Avenue du Recteur Pineau, 86022 Poitiers (France)
Search for more papers by this authorWe thank Prof. Jean-Pierre Gesson (Poitiers) for many useful discussions and the EPSRC National Mass Spectrometry Service Centre (Swansea, UK) for accurate mass data. This work was supported by the Science and Technology Department of the French Embassy in the United Kingdom, the Scottish Executive and the Royal Society of Edinburgh. D.A.L. is an EPSRC Senior Research Fellow and holds a Royal Society-Wolfson Research Merit Award.
Graphical Abstract
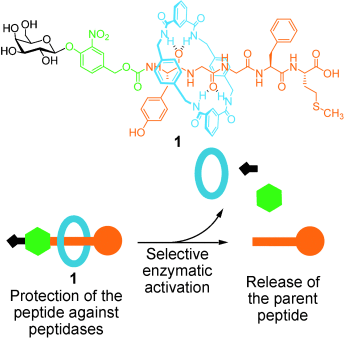
Ring of protection: A [2]rotaxane 1 protects and selectively releases a bioactive pentapeptide. The rotaxane macrocycle provides a defensive shield that very significantly improves the poor stability of the peptide to both individual peptidases and the cocktail of enzymes present in human plasma. Glycosidase-catalyzed cleavage of a carbohydrate ‘stopper’ in the rotaxane triggers release of the parent peptide (see picture).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200903215_sm_miscellaneous_information.pdf928.6 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. D. Nguyen, H.-R. Tseng, P. C. Celestre, A. H. Flood, Y. Liu, J. F. Stoddart, J. I. Zink, Proc. Natl. Acad. Sci. USA 2005, 102, 10029–10034;
- 1bK. C.-F. Leung, T. D. Nguyen, J. F. Stoddart, J. I. Zink, Chem. Mater. 2006, 18, 5919–5928;
- 1cT. D. Nguyen, K. C.-F. Leung, M. Liong, C. D. Pentecost, J. F. Stoddart, J. I. Zink, Org. Lett. 2006, 8, 3363–3366;
- 1dT. D. Nguyen, Y. Liu, S. Saha, K. C.-F. Leung, J. F. Stoddart, J. I. Zink, J. Am. Chem. Soc. 2007, 129, 626–634;
- 1eT. D. Nguyen, K. C.-F. Leung, M. Liong, Y. Liu, J. F. Stoddart, J. I. Zink, Adv. Funct. Mater. 2007, 17, 2101–2110;
- 1fS. Angelos, Y.-W. Yang, K. Patel, J. F. Stoddart, J. I. Zink, Angew. Chem. 2008, 120, 2254–2258;
10.1002/ange.200705211 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2222–2226;
- 1gK. Patel, S. Angelos, W. R. Ditchel, A. Coskun, Y.-W. Yang, J. I. Zink, J. F. Stoddart, J. Am. Chem. Soc. 2008, 130, 2382–2383;
- 1hD. P. Ferris, Y.-L. Zhao, N. M. Khashab, H. A. Khatib, J. F. Stoddart, J. I. Zink, J. Am. Chem. Soc. 2009, 131, 1686–1688.
- 2
- 2aE. Arunkumar, C. C. Forbes, B. C. Noll, B. D. Smith, J. Am. Chem. Soc. 2005, 127, 3288–3289;
- 2bE. Arunkumar, N. Fu, B. D. Smith, Chem. Eur. J. 2006, 12, 4684–4690;
- 2cJ. R. Johnson, N. Fu, E. Arunkumar, W. M. Leevy, S. T. Gammon, D. Piwnica-Worms, B. D. Smith, Angew. Chem. 2007, 119, 5624–5627;
10.1002/ange.200701491 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5528–5531;
- 2dJ. J. Gassensmith, E. Arunkumar, L. Barr, J. M. Baumes, K. M. DiVittorio, J. R. Johnson, B. C. Noll, B. D. Smith, J. Am. Chem. Soc. 2007, 129, 15054–15059.
- 3A. G. Cheetham, M. G. Hutchings, T. D. W. Claridge, H. L. Anderson, Angew. Chem. 2006, 118, 1626–1629;
10.1002/ange.200504064 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1596–1599.
- 4
- 4aV. Dvornikovs, B. E. House, M. Kaetzel, J. R. Dedman, D. B. Smithrud, J. Am. Chem. Soc. 2003, 125, 8290–8301;
- 4bX. Bao, I. Isaacsohn, A. F. Drew, D. B. Smithrud, J. Am. Chem. Soc. 2006, 128, 12229–12238;
- 4cJ. Zhu, B. E. House, E. Fleck, I. Isaacsohn, A. F. Drew, D. B. Smithrud, Bioorg. Med. Chem. Lett. 2007, 17, 5058–5062;
- 4dX. Wang, X. Bao, M. McFarland-Mancini, I. Isaacsohn, A. F. Drew, D. B. Smithrud, J. Am. Chem. Soc. 2007, 129, 7284–7293;
- 4eJ. Zhu, M. McFarland-Mancini, A. F. Drew, D. B. Smithrud, Bioorg. Med. Chem. Lett. 2009, 19, 520–523.
- 5
- 5aT. Ooya, N. Yui, Crit. Rev. Ther. Drug Carrier Syst. 1999, 16, 289–330;
- 5bT. Ooya, N. Yui, J. Controlled Release 1999, 58, 251–269;
- 5cT. Ooya, K. Arizono, N. Yui, Polym. Adv. Technol. 2000, 11, 642–651;
- 5dM. Eguchi, T. Ooya, N. Yui, J. Controlled Release 2004, 96, 301–307.
- 6
- 6aD. A. Leigh, A. Murphy, J. P. Smart, A. M. Z. Slawin, Angew. Chem. 1997, 109, 752–756;
10.1002/ange.19971090711 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 728–732;
- 6bG. W. H. Wurpel, A. M. Brouwer, I. H. M. van Stokkum, A. Farran, D. A. Leigh, J. Am. Chem. Soc. 2001, 123, 11327–11328;
- 6cG. Brancato, F. Coutrot, D. A. Leigh, A. Murphy, J. K. Y. Wong, F. Zerbetto, Proc. Natl. Acad. Sci. USA 2002, 99, 4967–4971;
- 6dM. Asakawa, G. Brancato, M. Fanti, D. A. Leigh, T. Shimizu, A. M. Z. Slawin, J. K. Y. Wong, F. Zerbetto, S. Zhang, J. Am. Chem. Soc. 2002, 124, 2939–2950;
- 6eT. Da Ros, D. M. Guldi, A. Farran Morales, D. A. Leigh, M. Prato, R. Turco, Org. Lett. 2003, 5, 689–691;
- 6fG. Bottari, D. A. Leigh, E. M. Pérez, J. Am. Chem. Soc. 2003, 125, 13360–13361;
- 6gJ. S. Hannam, S. M. Lacy, D. A. Leigh, C. G. Saiz, A. M. Z. Slawin, S. G. Stitchell, Angew. Chem. 2004, 116, 3322–3326;
10.1002/ange.200353606 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3260–3264;
- 6hD. A. Leigh, M. Á. F. Morales, E. M. Pérez, J. K. Y. Wong, C. G. Saiz, A. M. Z. Slawin, A. J. Carmichael, D. M. Haddleton, A. M. Brouwer, W. J. Buma, G. W. H. Wurpel, S. León, F. Zerbetto, Angew. Chem. 2005, 117, 3122–3127;
10.1002/ange.200500101 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3062–3067.
- 7 Peptide-Based Drug Design Methods in Molecular Biology, Vol. 494 (Ed.: ), Humana Press, New York, 2008.
- 8This is likely a result of the folding of the thread through strong intramolecular hydrogen bonding in the nonpolar solvents used to promote hydrogen bond-template rotaxane formation. Yields of rotaxanes from related pentapeptide threads attempted in our laboratory were typically less than 1 %.
- 9P. Garner, J. U. Yoo, Tetrahedron Lett. 1993, 34, 1275–1278.
- 10
- 10aS. J. Rowan, J. F. Stoddart, J. Am. Chem. Soc. 2000, 122, 164–165;
- 10bD. W. Zehnder, D. B. Smithrud, Org. Lett. 2001, 3, 2485–2487;
- 10cS.-H. Chiu, S. J. Rowan, S. J. Cantrill, J. F. Stoddart, A. J. P. White, D. J. Williams, Chem. Eur. J. 2002, 8, 5170–5183;
10.1002/1521-3765(20021115)8:22<5170::AID-CHEM5170>3.0.CO;2-S CAS PubMed Web of Science® Google Scholar
- 10dT. J. Kidd, T. J. A. Loontjens, D. A. Leigh, J. K. Y. Wong, Angew. Chem. 2003, 115, 3501–3505;
10.1002/ange.200351458 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3379–3383;
- 10eO. Š. Miljanić, W. R. Dichtel, I. Aprahamian, R. D. Rohde, H. D. Agnew, J. R. Heath, J. F. Stoddart, QSAR Comb. Sci. 2007, 26, 1165–1174;
- 10fJ. M. Spruell, W. R. Dichtel, J. R. Heath, J. F. Stoddart, Chem. Eur. J. 2008, 14, 4168–4177.
- 11
- 11aI. S. Zagon, P. J. McLaughlin, Neuropeptides 2003, 37, 79–88;
- 11bJ. Fichna, A. Janecka, Cancer Metastasis Rev. 2004, 23, 351–366;
- 11cI. S. Zagon, P. J. McLaughlin, Neuropeptides 2005, 39, 495–505;
- 11dI. S. Zagon, K. A. Rahn, P. J. McLaughlin, Neuropeptides 2007, 41, 441–452;
- 11eF. Cheng, I. S. Zagon, M. F. Verderame, P. J. McLaughlin, Cancer Res. 2007, 67, 10511–10518;
- 11fF. Cheng, P. J. McLaughlin, M. F. Verderame, I. S. Zagon, Mol. Biol. Cell 2009, 20, 319–327.
- 12
- 12aH. Cheng, X. Cao, M. Xian, L. Fang, T. B. Cai, J. J. Ji, J. B. Tunac, D. Sun, P. G. Wang, J. Med. Chem. 2005, 48, 645–652;
- 12bL. F. Tietze, F. Major, I. Schuberth, Angew. Chem. 2006, 118, 6724–6727;
10.1002/ange.200600936 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6574–6577;
- 12cA. Kamal, V. Tekumalla, A. Krishnan, M. Pal-Bhadra, U. Bhadra, ChemMedChem 2008, 3, 794–802.
- 13
- 13aP. D. Senter, C. J. Springer, Adv. Drug Delivery Rev. 2001, 53, 247–264;
- 13bK. D. Bagshawe, Expert Rev. Anticancer Ther. 2006, 6, 1421–1431.
- 14
- 14aS. Papot, I. Tranoy, F. Tillequin, J.-C. Florent, J.-P. Gesson, Curr. Med. Chem. Anti-Cancer Agents 2002, 2, 155–185;
- 14bI. Tranoy-Opalinski, A. Fernandes, M. Thomas, J.-P. Gesson, S. Papot, Anti-Cancer Agents Med. Chem. 2008, 8, 618–637.
- 15
- 15aF. Coutrot, E. Busseron, J.-L. Montero, Org. Lett. 2008, 10, 753–756;
- 15bF. Coutrot, C. Romuald, E. Busseron, Org. Lett. 2008, 10, 3741–3744;
- 15cF. Coutrot, E. Busseron, Chem. Eur. J. 2008, 14, 4784–4787;
- 15dF. Coutrot, E. Busseron, Chem. Eur. J. 2009, 15, 5186–5190.
- 16
- 16aC. Gros, B. Giros, J. C. Schwartz, Biochemistry 1985, 24, 2179–2185;
- 16bH. Terashima, N. Bunnett, J. Gastroenterol. 1995, 30, 696–704.
- 17J. Lanzillo, J. Stevens, Y. Dasarathy, H. Yotsumoto, B. Fanburg, J. Biol. Chem. 1985, 260, 14938–14944.
- 18Intermediates 8 and 9 are only stable for a few minutes at pH 7 at 37 °C. However, they survive for hours at pH 2 at room temperature (the equilibrium between the phenol and phenolate forms is shifted toward the phenol and elimination occurs only slowly). In the β-gal-catalyzed reactions, aliquots were withdrawn from the reaction medium and diluted with a solution of trifluoroacetic acid (to pH 2) before being injected into the HPLC apparatus with the mobile phase buffered at pH 2 (see the Supporting Information).