pH-Regulated Asymmetric Transfer Hydrogenation of Quinolines in Water†
Chao Wang
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorChaoqun Li
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorXiaofeng Wu Dr.
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorAlan Pettman Dr.
Chemical R & D, Global Research & Development, Pfizer, Sandwich, Kent CT13 9NJ (UK)
Search for more papers by this authorJianliang Xiao Prof.
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorChao Wang
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorChaoqun Li
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorXiaofeng Wu Dr.
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorAlan Pettman Dr.
Chemical R & D, Global Research & Development, Pfizer, Sandwich, Kent CT13 9NJ (UK)
Search for more papers by this authorJianliang Xiao Prof.
Liverpool Centre for Materials and Catalysis, Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK), Fax: (+44) 151-794-3589 http://pcwww.liv.ac.uk/∼jxiao
Search for more papers by this authorWe are grateful to Pfizer for funding (C.W.) and AstraZeneca for support.
Graphical Abstract
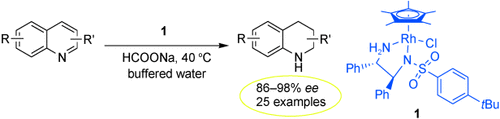
In buffered water, a broad range of quinoline derivatives underwent asymmetric transfer hydrogenation in air with the rhodium catalyst 1 and sodium formate as the hydrogen source to furnish synthetically important 1,2,3,4-tetrahydroquinolines with excellent enantioselectivities (see scheme; R=H, Me, F, Cl, Br, OMe; R′=alkyl, aryl).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200902570_sm_miscellaneous_information.pdf2.6 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. R. Katritzky, S. Rachwal, B. Rachwal, Tetrahedron 1996, 52, 15031.
- 2
- 2aJ. H. Rakotoson, N. Fabre, I. Jacquemond-Collet, S. Hannedouche, I. Fourasté, C. Moulis, Planta Med. 1998, 64, 762;
- 2bI. Jacquemond-Collet, S. Hannedouche, N. Fabre, I. Fourasté, C. Moulis, Phytochemistry 1999, 51, 1167.
- 3P. J. Houghton, T. Z. Woldemariam, Y. Watanabe, M. Yates, Planta Med. 1999, 65, 250.
- 4For examples of the asymmetric hydrogenation of quinolines, see:
- 4aW. B. Wang, S. M. Lu, P. Y. Yang, X. W. Han, Y. G. Zhou, J. Am. Chem. Soc. 2003, 125, 10536;
- 4bL. J. Xu, K. H. Lam, J. X. Ji, J. Wu, Q. H. Fan, W. H. Lo, A. S. C. Chan, Chem. Commun. 2005, 1390;
- 4cM. T. Reetz, X. G. Li, Chem. Commun. 2006, 2159;
- 4dS. M. Lu, C. Bolm, Adv. Synth. Catal. 2008, 350, 1101;
- 4eN. Mršić, L. Lefort, J. A. F. Boogers, A. J. Minnaard, B. L. Feringa, J. G. de Vries, Adv. Synth. Catal. 2008, 350, 1081;
- 4fZ. W. Li, T. L. Wang, Y. M. He, Z. J. Wang, Q. H. Fan, J. Pan, L. J. Xu, Org. Lett. 2008, 10, 5265;
- 4gH. Zhou, Z. Li, Z. Wang, T. Wang, L. Xu, Y. He, Q. H. Fan, J. Pan, L. Gu, A. S. C. Chan, Angew. Chem. 2008, 120, 8592; Angew. Chem. Int. Ed. 2008, 47, 8464;
- 4hD. W. Wang, X. B. Wang, D. S. Wang, S. M. Lu, Y. G. Zhou, Y. X. Li, J. Org. Chem. 2009, 74, 2780.
- 5
- 5aM. Rueping, A. P. Antonchick, T. Theissmann, Angew. Chem. 2006, 118, 3765;
10.1002/ange.200600191 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3683;
- 5bM. Rueping, T. Theissmann, S. Raja, J. W. Bats, Adv. Synth. Catal. 2008, 350, 1001;
- 5cQ. S. Guo, D. M. Du, J. Xu, Angew. Chem. 2008, 120, 771; Angew. Chem. Int. Ed. 2008, 47, 759.
- 6For reviews on ATH, see:
- 6aR. Noyori, S. Hashiguchi, Acc. Chem. Res. 1997, 30, 97;
- 6bM. J. Palmer, M. Wills, Tetrahedron: Asymmetry 1999, 10, 2045;
- 6cK. Everaere, A. Mortreux, J. F. Carpentier, Adv. Synth. Catal. 2003, 345, 67;
- 6dS. Gladiali, E. Alberico, Chem. Soc. Rev. 2006, 35, 226;
- 6eS. E. Clapham, A. Hadzovic, R. H. Morris, Coord. Chem. Rev. 2004, 248, 2201;
- 6fJ. S. M. Samec, J. E. Bäckvall, P. G. Andersson, P. Brandt, Chem. Soc. Rev. 2006, 35, 237;
- 6gX. F. Wu, J. L. Xiao, Chem. Commun. 2007, 2449;
- 6hT. Ikariya, A. J. Blacker, Acc. Chem. Res. 2007, 40, 1300;
- 6iC. Wang, X. F. Wu, J. L. Xiao, Chem. Asian J. 2008, 3, 1750.
- 7For examples of the non-asymmetric transfer hydrogenation of quinolines, see:
- 7aK. Fujita, C. Kitatsuji, S. Furukawa, R. Yamaguchi, Tetrahedron Lett. 2004, 45, 3215;
- 7bA. M. Voutchkova, D. Gnanamgari, C. E. Jakobsche, C. Butler, S. J. Miller, J. Parr, R. H. Crabtree, J. Organomet. Chem. 2008, 693, 1815.
- 8An iridium-catalyzed ATH of quinolines with the Hantzsch ester in toluene/dioxane was reported recently (10–87 % ee): D. W. Wang, W. Zeng, Y. G. Zhou, Tetrahedron: Asymmetry 2007, 18, 1103.
- 9For recent reviews on organic reactions in water, see:
- 9aU. M. Lindström, Chem. Rev. 2002, 102, 2751;
- 9bS. Kobayashi, K. Manabe, Acc. Chem. Res. 2002, 35, 209;
- 9cC. J. Li, Chem. Rev. 2005, 105, 3095;
- 9dT. Dwars, E. Paetzold, G. Oehme, Angew. Chem. 2005, 117, 7338;
10.1002/ange.200501365 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7174;
- 9eA. Chanda, V. V. Fokin, Chem. Rev. 2009, 109, 725.
- 10
- 10aS. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb, K. B. Sharpless, Angew. Chem. 2005, 117, 3339; Angew. Chem. Int. Ed. 2005, 44, 3275;
- 10bX. F. Wu, J. K. Liu, X. H. Li, A. Zanotti-Gerosa, F. Hancock, D. Vinci, J. W. Ruan, J. L. Xiao, Angew. Chem. 2006, 118, 6870; Angew. Chem. Int. Ed. 2006, 45, 6718;
- 10cN. Mase, Y. Nakai, N. Ohara, H. Yoda, K. Takabe, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 734.
- 11
- 11aX. F. Wu, X. G. Li, W. Hems, F. King, J. L. Xiao, Org. Biomol. Chem. 2004, 2, 1818;
- 11bX. F. Wu, X. G. Li, F. King, J. L. Xiao, Angew. Chem. 2005, 117, 3473; Angew. Chem. Int. Ed. 2005, 44, 3407;
- 11cX. F. Wu, X. H. Li, A. Zanotti-Gerosa, A. Pettman, J. K. Liu, A. J. Mills, J. L. Xiao, Chem. Eur. J. 2008, 14, 2209.
- 12aC. Bubert, J. Blacker, S. M. Brown, J. Crosby, S. Fitzjohn, J. P. Muxworthy, T. Thorpe, J. M. J. Williams, Tetrahedron Lett. 2001, 42, 4037;
- 12bH. Y. Rhyoo, H. J. Park, Y. K. Chung, Chem. Commun. 2001, 2064;
- 12cP. N. Liu, J. G. Deng, Y. Q. Tu, S. H. Wang, Chem. Commun. 2004, 2070;
- 12dJ. Canivet, G. Labat, H. Stoeckli-Evans, G. Süss-Fink, Eur. J. Inorg. Chem. 2005, 4493;
- 12eD. S. Matharu, D. J. Morris, G. J. Clarkson, M. Wills, Chem. Commun. 2006, 3232;
- 12fC. Letondor, A. Pordea, N. Humbert, A. Ivanova, S. Mazurek, M. Novic, T. R. Ward, J. Am. Chem. Soc. 2006, 128, 8320;
- 12gD. M. Jiang, J. S. Gao, Q. H. Yang, J. Yang, C. Li, Chem. Mater. 2006, 18, 6012;
- 12hH. F. Zhou, Q. H. Fan, Y. Y. Huang, L. Wu, Y. M. He, W. J. Tang, L. Q. Gu, A. S. C. Chan, J. Mol. Catal. A 2007, 275, 47;
- 12iS. Itsuno, M. Takahashi, Y. Arakawa, N. Haraguchi, Pure Appl. Chem. 2007, 79, 1471;
- 12jA. Schlatter, W. D. Woggon, Adv. Synth. Catal. 2008, 350, 995;
- 12kK. Ahlford, J. Lind, L. Mäler, H. Adolfsson, Green Chem. 2008, 10, 832.
- 13For examples of the ATH of imines in water, see:
- 13aJ. S. Wu, F. Wang, Y. P. Ma, X. Cui, L. F. Cun, J. Zhu, J. G. Deng, B. L. Yu, Chem. Commun. 2006, 1766;
- 13bJ. Canivet, G. Süss-Fink, Green Chem. 2007, 9, 391.
- 14S. Hashiguchi, A. Fujii, J. Takehara, T. Ikariya, R. Noyori, J. Am. Chem. Soc. 1995, 117, 7562.
- 15K. Mashima, T. Abe, K. Tani, Chem. Lett. 1998, 1199.
- 16For examples of mechanistic studies of imine reduction, see:
- 16aD. G. Blackmond, M. Ropic, M. Stefinvic, Org. Process Res. Dev. 2006, 10, 457;
- 16bJ. B. Åberg, J. S. M. Samec, J. E. Bäckvall, Chem. Commun. 2006, 2771;
- 16cC. P. Casey, T. B. Clark, I. A. Guzei, J. Am. Chem. Soc. 2007, 129, 11821;
- 16dJ. E. D. Martins, G. J. Clarkson, M. Wills, Org. Lett. 2009, 11, 847.
- 17
- 17aH. Guan, M. Iimura, M. P. Magee, J. R. Norton, G. Zhu, J. Am. Chem. Soc. 2005, 127, 7805;
- 17bT. Ohkuma, N. Utsumi, K. Tsutsumi, K. Murata, C. Sandoval, R. Noyori, J. Am. Chem. Soc. 2006, 128, 8724.
- 18
- 18aC. Q. Li, C. Wang, B. Villa-Marcos, J. L. Xiao, J. Am. Chem. Soc. 2008, 130, 14450;
- 18bC. Q. Li, B. Villa-Marcos, J. L. Xiao, J. Am. Chem. Soc. 2009, 131, 6967.
- 19The buffer capacities of a 2 M HCOOH/HCOONa solution and a 2 M HOAc/NaOAc solution (pH 5) are 0.21 and 1.07, respectively.