[Zn6Sn3Bi8]4−: Expanding the Intermetalloid Zintl Anion Concept to Ternary Systems†
Felicitas Lips Dipl.-Chem.
Fachbereich Chemie, Wissenschaftliches Zentrum für Materialwissenschaften (WZMW), Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35043 Marburg (Germany), Fax: (+49) 6421-282-5653
Search for more papers by this authorStefanie Dehnen Prof. Dr.
Fachbereich Chemie, Wissenschaftliches Zentrum für Materialwissenschaften (WZMW), Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35043 Marburg (Germany), Fax: (+49) 6421-282-5653
Search for more papers by this authorFelicitas Lips Dipl.-Chem.
Fachbereich Chemie, Wissenschaftliches Zentrum für Materialwissenschaften (WZMW), Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35043 Marburg (Germany), Fax: (+49) 6421-282-5653
Search for more papers by this authorStefanie Dehnen Prof. Dr.
Fachbereich Chemie, Wissenschaftliches Zentrum für Materialwissenschaften (WZMW), Philipps-Universität Marburg, Hans-Meerwein-Strasse, 35043 Marburg (Germany), Fax: (+49) 6421-282-5653
Search for more papers by this authorThis work was supported by the Fonds der Chemischen Industrie (Chemiefonds-Stipendium for F.L.)
Graphical Abstract
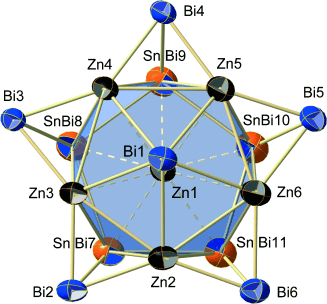
Zintl to the third: [Zn§Zn5Sn3Bi3§Bi5]4− (see structure), the first ternary intermetalloid Zintl anion, was obtained upon reaction of the binary anion [Sn2Bi2]2− with ZnPh2 in 1,2-diaminoethane/[2.2.2]crypt solution. X-ray structure analysis and DFT calculations indicate varied bonding within the intermetalloid cage and rationalize the impact of the ternary composition on structural and electronic properties.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200902249_sm_miscellaneous_information.pdf3.6 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Joannis, Ann. Chim. Phys. 1906, 7, 75;
- 1bC. A. Kraus, J. Am. Chem. Soc. 1925, 47, 43–60.
- 2E. Zintl, J. Goubeau, W. Dullenkopf, J. Phys. Chem. 1931, A154, 1–46.
10.1515/zpch-1931-15402 Google Scholar
- 3
- 3aL. Diehl, K. Khodadadeh, D. Kummer, J. Strähle, Chem. Ber. 1976, 109, 3404–3418;
- 3bP. A. Edwards, J. D. Corbett, Inorg. Chem. 1977, 16, 903–907.
- 4
- 4aS. C. Sevov, J. M. Goicoechea, Organometallics 2006, 25, 5678–5692;
- 4bT. F. Fässler, Angew. Chem. 2001, 113, 4289–4293;
10.1002/1521-3757(20011119)113:22<4289::AID-ANGE4289>3.0.CO;2-E Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4161–4165;10.1002/1521-3773(20011119)40:22<4161::AID-ANIE4161>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 4cJ. D. Corbett, Angew. Chem. 2000, 112, 682–704;
10.1002/(SICI)1521-3757(20000218)112:4<682::AID-ANGE682>3.0.CO;2-3 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 670–690;10.1002/(SICI)1521-3773(20000218)39:4<670::AID-ANIE670>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 4dJ. D. Corbett, Chem. Rev. 1985, 85, 383–397.
- 5T. F. Fässler, Coord. Chem. Rev. 2001, 215, 347–377.
- 6
- 6aT. F. Fässler, S. D. Hoffmann, Angew. Chem. 2004, 116, 6400–6406;
10.1002/ange.200460427 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6242–6247;
- 6bN. Korber, Angew. Chem. 2009, 121, 3262–3264;
10.1002/ange.200900133 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3216–3217.
- 7 J. D. Corbett, Angew. Chem. 2000, 112, 682–704;
10.1002/(SICI)1521-3757(20000218)112:4<682::AID-ANGE682>3.0.CO;2-3 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 670–690.10.1002/(SICI)1521-3773(20000218)39:4<670::AID-ANIE670>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 8S. Scharfe, T. F. Fässler, S. Stegmaier, S. D. Hoffman, K. Ruhland, Chem. Eur. J. 2008, 14, 4479–4483.
- 9B. Kesanli, J. E. Halsig, P. Zavalij, J. C. Fettinger, Y.-F. Lam, B. W. Eichhorn, J. Am. Chem. Soc. 2007, 129, 4567–4574.
- 10
- 10aJ. M. Goicoechea, S. C. Sevov, J. Am. Chem. Soc. 2005, 127, 7676–7677;
- 10bZ.-M. Sun, H. Xiao, J. Li, L.-S. Wang, J. Am. Chem. Soc. 2007, 129, 9560–9561;
- 10cF. S. Kocak, P. Zavalij, Y.-F. Lam, B. W. Eichhorn, Inorg. Chem. 2008, 47, 3515–3520.
- 11aJ. M. Goicoechea, S. C. Sevov, Angew. Chem. 2005, 117, 4094–4096;
10.1002/ange.200500600 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4026–4028;
- 11bJ. M. Goicoechea, S. C. Sevov, Angew. Chem. 2006, 118, 5271–5274;
10.1002/ange.200601805 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5147–5150.
- 12aM. J. Moses, J. C. Fettinger, B. W. Eichorn, Inorg. Chem. 2007, 46, 1036–1038;
- 12bM. J. Moses, J. C. Fettinger, B. W. Eichhorn, Science 2003, 300, 778–780.
- 13B. Zhou, M. S. Denning, D. L. Kays, J. M. Goicoechea, J. Am. Chem. Soc. 2009, 131, 2802–2803.
- 14J.-Q Wang, S. Stegmaier, T. F. Fässler, Angew. Chem. 2009, 121, 2032–2036; Angew. Chem. Int. Ed. 2009, 48, 1998–2002.
- 15
- 15aK. Wade, Adv. Inorg. Chem. Radiochem. 1976, 18, 1–67;
- 15bD. M. P. Mingos, Nature Phys. Sci. 1972, 236, 99–102;
- 15cD. M. P. Mingos, Acc. Chem. Res. 1984, 17, 311–319.
- 16
- 16aA. M. Guloy, R. Ramlau, Z. Tang, W. Schnelle, M. Baitinger, Y. Grin, Nature 2006, 443, 320–323;
- 16bM. G. Kanatzidis, G. S. Armatas, Science 2006, 313, 817–820;
- 16cD. Sun, A. E. Riley, A. J. Cadby, E. K. Richmann, S. D. Korlann, S. H. Tolbert, Nature 2006, 441, 1126–1130;
- 16dT. F. Fässler, Angew. Chem. 2007, 119, 2624–2628;
10.1002/ange.200604586 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2572–2575.
- 17
- 17aM. Baitinger, Y. Grin, R. Kniep, H. G. von Schnering, Z. Kristallogr. 1999, 214, 453–454;
- 17bI. F. Hewaidy, E. Busmann, W. Klemm, Z. Anorg. Allg. Chem. 1964, 328, 283–293;
- 17cH. G. von Schnering, J. Llanos, J.-H. Chang, K. Peters, E. M. Peters, R. Nesper, Z. Kristallogr. 2005, 220, 324–326;
- 17dE. Busmann, Z. Anorg. Allg. Chem. 1961, 313, 90–106.
- 18
- 18aR. W. Rudolph, W. L. Wilson, R. C. Taylor, J. Am. Chem. Soc. 1981, 103, 2480–2481;
- 18bS. C. Critchlow, J. D. Corbett, J. Chem. Soc. Chem. Commun. 1981, 236–237;
- 18cK. Wiesler, K. Brandl, A. Fleischmann, N. Korber, Z. Anorg. Allg. Chem. 2009, 635, 508–512.
- 19S. Dehnen, M. Melullis, Coord. Chem. Rev. 2007, 251, 1259–1280.
- 20[2.2.2]crypt: 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane.
- 21S. C. Critchlow, J. D. Corbett, Inorg. Chem. 1982, 21, 3286–3290.
- 22X-ray data for C76.5H152N9O24K4Zn6Sn3Bi8 (1): monoclinic, P21/n, a=15.442(3), b=28.506(6), c=27.937(6) Å, β=90.47(3), V=12 297(4) Å3, Z=4, R1 (I>2 σ(I))=0.0582, wR2 (all data)=0.1022, GooF (all data) 0.950, max. peak/hole 1.24/−2.42. X-ray data for C45H88N6O12K2Sn6.96Bi2.04 (2): hexagonal, P63/m, a=12.1635(17), c=26.336(5) Å, V=3374.4(10) Å3, Z=2, R1 (I>2 σ(I))=0.0562, wR2 (all data)=0.1451, GooF (all data)=1.112, max peak/hole 2.09/−1.11. For more details, see the Supporting Information. CCDC 733155 (1) and 733156 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 23W. Sheldrick, SHELXTL 5.1, Bruker AXS Inc., 6300 Enterprise Lane, Madison, 1997.
- 24
- 24aR. G. Parr, W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York, 1988;
- 24bT. Ziegler, Chem. Rev. 1991, 91, 651–667.
- 25Program system TURBOMOLE:
- 25aR. Ahlrichs, M. Bär, M. Häser, H. Horn, C. Kölmel, Chem. Phys. Lett. 1989, 162, 165–169;
- 25bO. Treutler, R. Ahlrichs, J. Chem. Phys. 1995, 102, 346–354.
- 26J. E. Huheey, E. A. Keiter, L. Keiter, Inorganic Chemistry: Principles of Structure and Reactivity, 4th ed., Harper Collins, New York, 1993.
- 27aA. E. Reed, R. B. Weinstock, F. Weinhold, J. Chem. Phys. 1985, 83, 735–746;
- 27bR. S. Mulliken, J. Chem. Phys. 1955, 23, 1833–1840.
- 28Natural charges of the external Bi atoms have been compared with those calculated for the reactant [Sn2Bi2]2− itself. The latter represents a tetrahedron with one external electron pair and a three-electron contribution to the tetrahedral cage per atom. The natural charges for [Sn2Bi2]2− are −0.49 (Bi) and −0.50 (Sn−), similar to the Sn and Bi atoms of the Zn6Sn3Bi2-centered pentagonal antiprism in 1. Thus, the five external Bi atoms exhibit a significantly lower electronic contribution to the cluster skeleton than would be expected for a three-electron donor. This conclusion is supported by a Mulliken population analysis[27b] that reveals that 69 % of all s and p electrons at the five external Bi atoms (Bi2–Bi6) do not contribute to the cluster bonding, which corresponds to between three (60 %) and four (80 %) of five electrons per Bi atom.
- 29
- 29aF. Gascoin, S. C. Sevov, Inorg. Chem. 2001, 40, 5177–5181;
- 29bL. Xu, S. Bobev, J. El-Bahraoui, S. C. Sevov, J. Am. Chem. Soc. 2000, 122, 1838–1839;
- 29cT. Hanauer, N. Korber, Z. Anorg. Allg. Chem. 2004, 630, 2532–2534;
- 29dA. Cisar, J. D. Corbett, Inorg. Chem. 1977, 16, 2482–2487;
- 29eA. N. Kuznetsov, T. F. Fässler, Z. Anorg. Allg. Chem. 2002, 628, 2537–2541.
- 30L. Xu, A. Ugrinov, S. C. Sevov, J. Am. Chem. Soc. 2001, 123, 4091–4092.
- 31
- 31aK. Eichkorn, O. Treutler, H. Öhm, M. Häser, R. Ahlrichs, Chem. Phys. Lett. 1995, 242, 652–660;
- 31bK. Eichkorn, F. Weigend, O. Treutler, R. Ahlrichs, Theor. Chim. Acta 1997, 97, 119–124.
- 32
- 32aA. D. Becke, Phys. Rev. A 1988, 38, 3098–3109;
- 32bS. H. Vosko, L. Wilk, M. Nusair, Can. J. Phys. 1980, 58, 1200–1205;
- 32cJ. P. Perdew, Phys. Rev. B 1986, 33, 8822–8837.
- 33F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297–3305.
- 34B. Metz, H. Stoll, M. Dolg, J. Chem. Phys. 2000, 113, 2563.
- 35
- 35aA. Klamt, G. Schürmann, J. Chem. Soc. Perkin Trans. 1 1993, 5, 799–805;
- 35bA. Schäfer, A. Klamt, D. Sattel, J. C. W. Lohrenz, F. Eckert, Phys. Chem. Chem. Phys. 2000, 2, 2187–2193.