Phosphonated Hexaphenylbenzene: A Crystalline Proton Conductor
Lucía Jiménez-García
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorAnke Kaltbeitzel Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorWojciech Pisula Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Present Address: Evonik Industries AG, Process Technology & Engineering, Process Technology-New Processes, Rodenbacher Chaussee 4, 63457 Hanau-Wolfgang (Germany)
Search for more papers by this authorJochen S. Gutmann Prof. Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorMarkus Klapper Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorKlaus Müllen Prof. Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorLucía Jiménez-García
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorAnke Kaltbeitzel Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorWojciech Pisula Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Present Address: Evonik Industries AG, Process Technology & Engineering, Process Technology-New Processes, Rodenbacher Chaussee 4, 63457 Hanau-Wolfgang (Germany)
Search for more papers by this authorJochen S. Gutmann Prof. Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorMarkus Klapper Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorKlaus Müllen Prof. Dr.
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz (Germany), Fax: (+49) 6131-379-350
Search for more papers by this authorGraphical Abstract
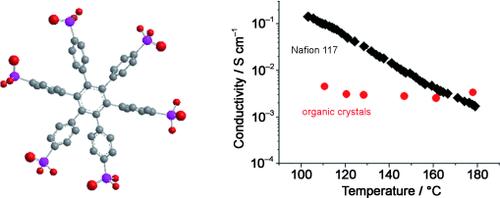
Well stacked: Organic crystals of small molecules constitute an alternative to common polymeric electrolytes (Nafion 117) and inorganic crystals that are employed as proton exchange membranes in fuel cell systems. A phosphonic acid containing hexaphenylbenzene forms a columnar supramolecular array and exhibits a high and constant intrinsic conductivity of 3.2×10−3 S cm−1 from 120 to 180 °C under 1 bar H2O atmosphere (see picture).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200902116_sm_miscellaneous_information.pdf284.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Pomes, Biol. Phys. 1999, 487, 194–200.
- 2M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla, J. E. McGrath, Chem. Rev. 2004, 104, 4587–4612.
- 3
- 3aK. D. Kreuer, J. Membr. Sci. 2001, 185, 29–39;
- 3bK. D. Kreuer, S. J. Paddison, E. Spohr, M. Schuster, Chem. Rev. 2004, 104, 4637–4678;
- 3cK. D. Kreuer, A. Rabenau, W. Weppner, Angew. Chem. 1982, 94, 224–225; Angew. Chem. Int. Ed. Engl. 1982, 21, 208–209.
- 4
- 4aM. Schuster, T. Rager, A. Noda, K. D. Kreuer, J. Maier, Fuel Cells 2005, 5, 355–365;
- 4bH. Steininger, M. Schuster, K. D. Kreuer, A. Kaltbeitzel, B. Bingöl, W. H. Meyer, S. Schauff, G. Brunklaus, J. Maier, H. W. Spiess, Phys. Chem. Chem. Phys. 2007, 9, 1764–1773;
- 4cB. Lafitte, P. Jannasch in Advances in Fuel Cells, Vol. 1 (Eds.: T. Zhao, K. D. Kreuer, T. V. Nguyen), Elsevier, Oxford, 2007, 119–185.
- 5
- 5aS. M. Haile, C. R. I. Chisholm, K. Sasaki, D. A. Boysen, T. Uda, Faraday Discuss. 2007, 134, 17–39;
- 5bD. Boysen, T. Uda, C. R. I. Chisholm, S. M. Haile, Science 2004, 303, 68–70;
- 5cS. M. Haile, D. A. Boysen, C. R. I. Chisholm, R. B. Merle, Nature 2001, 410, 910–913.
- 6
- 6aR. B. Merle, C. R. I. Chisholm, D. A. Boysen, S. M. Haile, Energy Fuels 2003, 17, 210–215.
- 7
- 7aM. Schuster, T. Rager, A. Noda, K. D. Kreuer, J. Maier, Fuel Cells 2005, 5, 355–365;
- 7bS. J. Paddison, K. D. Kreuer, J. Maier, Phys. Chem. Chem. Phys. 2006, 8, 4530–4542.
- 8
- 8aK. Hinokuma, M. Ata, Chem. Phys. Lett. 2001, 341, 442–446;
- 8bY. M. Li, K. Hinokuma, Solid State Ionics 2002, 150, 309–315;
- 8cM. Yamada, I. Honma, Chem. Phys. Lett. 2005, 402, 324–328;
- 8dI. Honma, M. Yamada, Bull. Chem. Soc. Jpn. 2007, 80, 2110–2123;
- 8eJ.-D. Kim, I. Honma, Solid State Ionics 2005, 176, 979–984.
- 9
- 9aJ. C. J. Bart, Acta Crystallogr. Sect. B 1968, 24, 1277–1287;
- 9bM. D. Watson, A. Fechtenkötter, K. Müllen, Chem. Rev. 2001, 101, 1267–1300.
- 10
- 10aK. Kobayashi, T. Shirasaka, A. Sato, E. Horn, N. Furukawa, Angew. Chem. 1999, 111, 3692–3694;
10.1002/(SICI)1521-3757(19991203)111:23<3692::AID-ANGE3692>3.0.CO;2-V Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3483–3485;10.1002/(SICI)1521-3773(19991203)38:23<3483::AID-ANIE3483>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 10bK. Kobayashi, T. Shirasaka, A. Sato, E. Horn, N. Furukawa, Tetrahedron Lett. 2000, 41, 89–93;
- 10cK. Kobayashi, A. Sato, S. Sakamoto, K. Yamaguchi, J. Am. Chem. Soc. 2003, 125, 3035–3045;
- 10dK. E. Maly, E. Gagnon, T. Maris, J. D. Wuest, J. Am. Chem. Soc. 2007, 129, 4306–4322.
- 11X. Feng, W. Pisula, L. Zhi, M. Takase, K. Müllen, Angew. Chem. 2008, 120, 1727; Angew. Chem. Int. Ed. 2008, 47, 1703–1706.
- 12R. Büll, Angew. Chem. 1936, 49, 145–158.
- 13
- 13aS. Hara, S. Takano, M. Miyayama, J. Phys. Chem. B 2004, 108, 5634–5639;
- 13bA. Kaltbeitzel, S. Schauff, H. Steininger, B. Bingöl, G. Brunklaus, W. H. Meyer, H. W. Spiess, Solid State Ionics 2007, 178, 469–474.
- 14
- 14aC. C. de Araujo, K. D. Kreuer, M. Schuster, G. Portale, H. Mendil-Jakani, G. Gebel, J. Maier, Phys. Chem. Chem. Phys. 2009, 11, 3305–3312;
- 14bM. Schuster, C. C. de Araujo, V. Atasanov, H. T. Andersen, K. D. Kreuer, J. Maier, Macromolecules 2009, 42, 3129–3137.
- 15Proton conductivity measurements for Nafion 117 were carried out in our equipment to allow a better comparison with p-6 PA-HPB. The values are in good agreement with reported data.
- 16This assumption is also supported by solid-state NMR studies: G. Brunklaus, B. Fassbender, W. H. Spiess, L. Jiménez-García, M. Klapper, K. Müllen, unpublished results.
- 17T. Rager, M. Schuster, H. Steininger, K. D. Kreuer, Adv. Mater. 2007, 19, 3317–3321.
- 18T. Uma, M. Nogami, Anal. Chem. 2008, 80, 506–508.
Citing Literature
December 21, 2009
Pages 9951-9953