Radicals and Transition-Metal Catalysis: An Alliance Par Excellence to Increase Reactivity and Selectivity in Organic Chemistry†
Leigh Ford Dr.
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo namesti 2, 16610 Prague 6 (Czech Republic), Fax: (+420) 220-183-578 http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorUllrich Jahn Priv.-Doz. Dr.
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo namesti 2, 16610 Prague 6 (Czech Republic), Fax: (+420) 220-183-578 http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorLeigh Ford Dr.
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo namesti 2, 16610 Prague 6 (Czech Republic), Fax: (+420) 220-183-578 http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorUllrich Jahn Priv.-Doz. Dr.
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo namesti 2, 16610 Prague 6 (Czech Republic), Fax: (+420) 220-183-578 http://www.uochb.cz/web/structure/616.html
Search for more papers by this authorWe gratefully acknowledge generous funding from the Institute of Organic Chemistry and Biochemistry of the Academy of Sciences of the Czech Republic.
Graphical Abstract
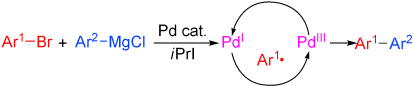
Welcome to radical catalysis: Radicals are “tamed” in transition-metal complexes which serve as catalysts for a number of reactions—Kumada couplings (see scheme), highly regioselective cobalt-catalyzed eliminations, Markovnikov additions, and reductive epoxide openings catalyzed by a titanium/rhodium system. All of these reactions are powered by the high reactivity of radical intermediates in the catalytic cycle of the metal-catalyzed process.
References
- 1B. Quiclet-Sire, S. Z. Zard, Pure Appl. Chem. 1997, 69, 645.
- 2 Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology, New Series, Group II, Vol. 13 (Ed.: ), Springer, Berlin, 1983.
- 3M. Newcomb, Tetrahedron 1993, 49, 1151.
- 4S. Z. Zard, Radical Reactions in Organic Synthesis, Oxford University Press, Oxford, 2003.
10.1093/oso/9780198502418.001.0001 Google Scholar
- 5
- 5a Metal-Catalyzed Cross-Coupling Reactions, 2nd ed. ), Wiley-VCH, Weinheim, 2004;
- 5b Transition Metals for Organic Synthesis (Eds.: ), Wiley-VCH, Weinhein, 2004.
- 6
- 6aQ. Y. Chen, Z. Y. Yang, C. X. Zhao, Z. M. Qui, J. Chem. Soc. Perkin Trans. 1 1988, 563;
- 6bD. P. Curran, C.-T. Chang, Tetrahedron Lett. 1990, 31, 933;
- 6cT. Ishiyama, S. Abe, N. Miyaura, A. Suzuki, Chem. Lett. 1992, 691;
- 6dH. Stadtmüller, A. Vaupel, C. E. Tucker, T. Stüdemann, P. Knochel, Chem. Eur. J. 1996, 2, 1204–1220.
- 7Reviews:
- 7aA. J. Clark, Chem. Soc. Rev. 2002, 31, 1;
- 7bT. Pintauer, K. Matyjaszewski, Chem. Soc. Rev. 2008, 37, 1087;
- 7cH. Matsumoto, T. Motegi, T. Nakano, Y. Nagai, J. Organomet. Chem. 1979, 174, 157, and references therein;
- 7dM. Kameyama, N. Kamigata, M. Kobayashi, J. Org. Chem. 1987, 52, 3312, and references therein.
- 8A. Rudolph, M. Lautens, Angew. Chem. 2009, 121, 2694;
10.1002/ange.200803611 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2656, and references therein.
- 9
- 9aI. Ryu, S. Kreimerman, F. Araki, S. Nishitani, Y. Oderaotoshi, S. Minakata, M. Komatsu, J. Am. Chem. Soc. 2002, 124, 3812; For applications see:
- 9bT. Fukuyama, S. Nishitani, T. Inouye, K. Morimoto, I. Ryu, Org. Lett. 2006, 8, 1383.
- 10M. W. Hooper, M. Utsunomiya, J. F. Hartwig, J. Org. Chem. 2003, 68, 2861.
- 11Leading reference: T. Hama, J. F. Hartwig, Org. Lett. 2008, 10, 1545.
- 12Z. Q. Weng, S. H. Teo, T. S. A. Hor, Acc. Chem. Res. 2007, 40, 676.
- 13G. Manolikakes, P. Knochel, Angew. Chem. 2009, 121, 211; Angew. Chem. Int. Ed. 2009, 48, 205.
- 14Generally the coupling of free aryl radicals is not synthetically useful. For a recent oxidative homocoupling of aryl Grignard reagents mediated or catalyzed by the free radical TEMPO, see: M. S. Maji, T. Pfeifer, A. Studer, Angew. Chem. 2008, 120, 9690; Angew. Chem. Int. Ed. 2008, 47, 9547. The mechanism has, however, not been elucidated.
- 15T. Kobayashi, H. Ohmiya, H. Yorimitsu, K. J. Oshima, J. Am. Chem. Soc. 2008, 130, 11276.
- 16
- 16aB. Gaspar, E. M. Carreira, Angew. Chem. 2008, 120, 5842;
10.1002/ange.200801760 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5758. Though no proof exists for the presence of radicals in this example, the proposed mechanism parallels that proposed and studied in detail for the hydrohydrazination and hydroazidation reactions. See: J. Waser, B. Gaspar, H. Nambu, E. M. Carreira, J. Am. Chem. Soc. 2006, 128, 11693.
- 17A. Gansäuer, C.-A. Fan, F. J. Piestert, J. Am. Chem. Soc. 2008, 130, 6916.
- 18R. S. Drago, J. G. Miller, M. A. Hoseton, R. D. Farris, M. J. Desmond, J. Am. Chem. Soc. 1991, 113, 4888.
- 19A. Bakac, L. M. Thomas, Inorg. Chem. 1996, 35, 5880.
- 20
- 20aT. D. Beeson, A. Mastracchio, J. B. Hong, K. Ashton, D. W. C. MacMillan, Science 2007, 316, 582;
- 20bD. A. Nicewicz, D. W. C. MacMillan, Science 2008, 322, 77; For a recent highlight:
- 20cP. Melchiorre, Angew. Chem. 2009, 121, 1386;
10.1002/ange.200804995 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1360.