The Challenge of Atropisomerism in Drug Discovery†
Jonathan Clayden Prof.
School of Chemistry, The University of Manchester, Oxford Road, Manchester M13 9PL (UK), Fax: (+44) 161-275-4939 http://clor2.ch.man.ac.uk/home.htm
Search for more papers by this authorWesley J. Moran Dr.
School of Chemistry, The University of Manchester, Oxford Road, Manchester M13 9PL (UK), Fax: (+44) 161-275-4939 http://clor2.ch.man.ac.uk/home.htm
Search for more papers by this authorPaul J. Edwards Dr.
Department of Chemistry, Boehringer-Ingelheim (Canada) Ltd. 2100 Cunard St., Laval, Québec, H7S 2G5 (Canada), Fax: (+450) 682-8434
Search for more papers by this authorSteven R. LaPlante Dr.
Department of Chemistry, Boehringer-Ingelheim (Canada) Ltd. 2100 Cunard St., Laval, Québec, H7S 2G5 (Canada), Fax: (+450) 682-8434
Search for more papers by this authorJonathan Clayden Prof.
School of Chemistry, The University of Manchester, Oxford Road, Manchester M13 9PL (UK), Fax: (+44) 161-275-4939 http://clor2.ch.man.ac.uk/home.htm
Search for more papers by this authorWesley J. Moran Dr.
School of Chemistry, The University of Manchester, Oxford Road, Manchester M13 9PL (UK), Fax: (+44) 161-275-4939 http://clor2.ch.man.ac.uk/home.htm
Search for more papers by this authorPaul J. Edwards Dr.
Department of Chemistry, Boehringer-Ingelheim (Canada) Ltd. 2100 Cunard St., Laval, Québec, H7S 2G5 (Canada), Fax: (+450) 682-8434
Search for more papers by this authorSteven R. LaPlante Dr.
Department of Chemistry, Boehringer-Ingelheim (Canada) Ltd. 2100 Cunard St., Laval, Québec, H7S 2G5 (Canada), Fax: (+450) 682-8434
Search for more papers by this authorWe are grateful to the EPSRC for funding. We would like to acknowledge critical discussions with P. Bonneau, L. Fader, J. Gillard, O. Hucke, A. Jakalian, N. Goudreau, and D. Krishnamurthy, as well as B. Lu for support in preparing this manuscript.
Graphical Abstract
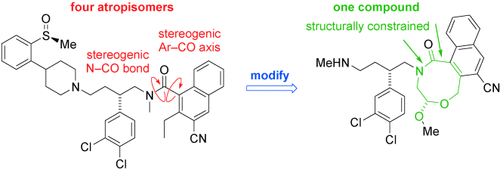
A twist in the tale: Recent reports have highlighted solutions to the problems encountered when drug candidates exist as slowly interconverting conformers or atropisomers (see scheme). This Minireview brings together the various strategies that have been adopted and proposes a general approach to handling an aspect of stereochemistry which has received little attention from drug regulatory agencies.
References
- 1
- 1aM. Eichelbaum, A. S. Gross, Adv. Drug Res. 1996, 28, 1;
- 1bR. Crossley, Chirality and the Biological Activity of Drugs, CRC, Boca Raton, FL, 1995;
- 1cR. R. Shah, J. M. Midgley, S. K. Branch, Adv. Drug React. Toxicol. Rev. 1998, 17, 145.
- 2
- 2aG. Blaschke, H. P. Kraft, K. Fickentscher, F. Kohler, Arzneim.-Forsch. 1979, 29, 1640;
- 2bS. Fabro, R. L. Smith, R. T. Williams, Nature 1967, 215, 296;
- 2cM. Reist, P. A. Carrupt, E. Francotte, B. Testa, Chem. Res. Toxicol. 1998, 11, 1521;
- 2dT. Eriksson, S. Bjorkman, B. Roth, A. Fyge, P. Hoglund, Chirality 1995, 7, 44.
- 3
- 3aI. Agranat, H. Caner, J. Caldwell, Nat. Rev. Drug Discovery 2002, 10, 753;
- 3bM. Rouhi, Chem. Eng. News 2003, 81, 56.
- 4
- 4aDepartment of Health & Human Services, Food and Drug Administration: FDA’s Policy Statement for the Development of New Stereoisomeric Drugs, Washington, DC, 1992: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm;
- 4bS. Miller, 18th International Symposium on Chirality, Busan, Korea, June 26, 2006: www.fda.gov/downloads/AboutFDA/CentersOffices/CDER/ucm103532.pd.
- 5
- 5aC. S. Chen, W. R. Shieh, P. H. Lu, S. Harriman, C. Y. Chen, Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1991, 1078, 411;
- 5bT. S. Tracy, S. D. Hall, Drug Metab Dispos 1992, 20, 322.
- 6aS. K. Branch, Chiral Separation Techniques. A Practical Approach, 2nd ed. ), Wiley-VCH, Weinheim, 2000, pp. 317–341;
10.1002/3527600361.ch13 Google Scholar
- 6bM. Strong, Food Drug Law J. 1999, 54, 463;
- 6cI. Agranat, H. Caner, Drug Discovery Today 1999, 4, 313.
- 7M. Oki, Top. Stereochem. 1983, 14, 1.
- 8G. Bringmann, A. J. P. Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. 2005, 117, 5518;
10.1002/ange.200462661 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5384.
- 9P. Eveleigh, E. C. Hulme, C. Schudt, N. J. M. Birdsall, Mol. Pharmacol. 1989, 35, 477.
- 10R. F. Friary, M. Spangler, R. Osterman, L. Schulman, J. H. Schwerdt, Chirality 1996, 8, 364.
- 11
- 11aJ. S. Albert, D. Aharony, D. Andisik, H. Barthlow, P. R. Bernstein, R. A. Bialecki, R. Dedinas, B. T. Dembofsky, D. Hill, K. Kirkland, G. M. Koether, B. J. Kosmider, C. Ohnmacht, W. Palmer, W. Potts, W. Rumsey, L. Shen, A. Shenvi, A. Sherwood, P. J. Warwick, K. Russell, J. Med. Chem. 2002, 45, 3972;
- 11bJ. S. Albert, C. Ohnmacht, P .R. Bernstein, W. Rumsey, D. Aharony, B. B. Masek, B. T. Dembofsky, G. M. Koether, W. Potts, J. L. Evenden, Tetrahedron 2004, 60, 4337.
- 12
- 12aR. A. Bragg, J. Clayden, G. A. Morris, J. H. Pink Chem. Eur. J. 2002, 8, 1279;
10.1002/1521-3765(20020315)8:6<1279::AID-CHEM1279>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 12bH. Iwamura, K. Mislow, Acc. Chem. Res. 1988, 21, 175.
- 13
- 13aS. D. Guile, J. R. Bantick, D. R. Cheshire, M. E. Cooper, A. M. Davis, D. K. Donald, R. Evans, C. Eyssade, D. D. Ferguson, S. Hill, R. Hutchinson, A. H. Ingall, L. P. Kingston, I. Martin, B. P. Martin, R. T. Mohammed, C. Murray, M. W. D. Perry, R. H. Reynolds, P. V. Thorne, D. J. Wilkinson, J. Withnall, Bioorg. Med. Chem. Lett. 2006, 16, 2260;
- 13bS. D. Guile, J. R. Bantick, M. E. Cooper, D. K. Donald, C. Eyssade, A. H. Ingall, R. J. Lewis, B. P. Martin, R. T. Mohammed, T. J. Potter, R. H. Reynolds, S. A. St-Gallay, A. D. Wright, J. Med. Chem. 2007, 50, 254.
- 14
- 14aA. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, D. Blythin, K. Cox, N. E. Wagner, J. Strizki, B. M. Baroudy, N. Dan, Bioorg. Med. Chem. Lett. 2003, 13, 705;
- 14bA. Palani, S. Shapiro, J. W. Clader, W. J. Greenlee, S. Vice, S. McCombie, K. Cox, J. Strizki, B. M. Baroudy, Bioorg. Med. Chem. Lett. 2003, 13, 709.
- 15Y. S. Zhou, L. K. Tay, D. Hughes, S. Donahue, J. Clin. Pharm. 2004, 44, 680.
- 16H. Tabata, K. Akiba, S. Lee, H. Takahashi, H. Natsugari, Org. Lett. 2009, 10, 4871.
- 17C. Deur, A. K. Agrawal, H. Baum, J. Booth, S. Bove, J. Brieland, A. Bunker, C. Connolly, J. Cornicelli, J. Dumin, B. Finzel, X. Gan, S. Guppy, G. Kamilar, K. Kilgore, P. Lee, C.-M. Loi, Z. Lou, M. Morris, L. Philippe, S. Przybranowski, F. Riley, B. Samas, B. Sanchez, H. Tecle, Z. Wang, K. Welch, M. Wilson, K. Yates, Bioorg. Med. Chem. Lett. 2007, 17, 4599.
- 18
- 18aC. Welch, M. Biba, P. Pye, R. Angelaud, M. Egbertson, J. Chromatogr. B 2008, 875, 118;
- 18bP. Cotelle, Expert Opin. Ther. Pat. 2009, 19, 87.
- 19J. Porter, A. Payne, I. Whitcombe, B. de Candole, D. Ford, R. Garlish, A. Hold, B. Hutchinson, G. Trevitt, J. Turner, C. Edwards, C. Watkins, J. Davis, C. Stubberfield, Bioorg. Med. Chem. Lett. 2009, 19, 1767.
- 20
- 20aF. Pietra, J. Phys. Org. Chem. 2007, 20, 1102;
- 20bD. L. Boger, S. Miyazaki, S. H. Kim, J. H. Wu, O. Loiseleur, S. L. Castle, J. Am. Chem. Soc. 1999, 121, 3226.