Multistep Synthesis Using Modular Flow Reactors: Bestmann–Ohira Reagent for the Formation of Alkynes and Triazoles†
Ian R. Baxendale Dr.
ITC, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK), Fax: (+44) 1223-336-362, http://leyitc.ch.cam.ac.uk/
Search for more papers by this authorSteven V. Ley Prof.
ITC, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK), Fax: (+44) 1223-336-362, http://leyitc.ch.cam.ac.uk/
Search for more papers by this authorAndrew C. Mansfield
Pfizer Global R&D Research Centre, Ramsgate Rd, Sandwich, Kent CT13 9NJ (UK)
Search for more papers by this authorChristopher D. Smith Dr.
ITC, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK), Fax: (+44) 1223-336-362, http://leyitc.ch.cam.ac.uk/
Search for more papers by this authorIan R. Baxendale Dr.
ITC, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK), Fax: (+44) 1223-336-362, http://leyitc.ch.cam.ac.uk/
Search for more papers by this authorSteven V. Ley Prof.
ITC, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK), Fax: (+44) 1223-336-362, http://leyitc.ch.cam.ac.uk/
Search for more papers by this authorAndrew C. Mansfield
Pfizer Global R&D Research Centre, Ramsgate Rd, Sandwich, Kent CT13 9NJ (UK)
Search for more papers by this authorChristopher D. Smith Dr.
ITC, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW (UK), Fax: (+44) 1223-336-362, http://leyitc.ch.cam.ac.uk/
Search for more papers by this authorWe gratefully acknowledge financial support from the EPRSC (to I.R.B.), Syngenta and Chemistry Innovation (to C.D.S.), the BP endowment (to S.V.L.) and Pfizer (to A.C.M.).
Graphical Abstract
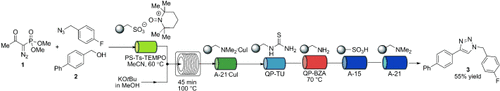
Multistep in flow: The Seyferth–Gilbert reagent 1 has been applied in a flow system to rapidly synthesize terminal alkynes. The system has been further applied to synthesize triazole 3 from alcohol 2 in a three-step oxidation/homologation/copper(I)-catalyzed azide–alkyne cycloaddition sequence without isolation of intermediates (see scheme).
Abstract
Multistep in flow: The Seyferth–Gilbert reagent 1 has been applied in a flow system to rapidly synthesize terminal alkynes. The system has been further applied to synthesize triazole 3 from alcohol 2 in a three-step oxidation/homologation/copper(I)-catalyzed azide–alkyne cycloaddition sequence without isolation of intermediates (see scheme).
References
- 1B. Hinzen, S. V. Ley, J. Chem. Soc. Perkin Trans. 1 1997, 1907–1908; B. Hinzen, S. V. Ley, J. Chem. Soc. Perkin Trans. 1 1998, 1–2; B. Hinzen, R. Lenz, S. V. Ley, Synthesis 1998, 977–979; S. V. Ley, M. H. Bolli, B. Hinzen, A.-G. Gervois, B. J. Hall, J. Chem. Soc. Perkin Trans. 1 1998, 2239–2242; S. V. Ley, M. H. Bolli, B. Hinzen, A.-G. Gervois, B. J. Hall, J. Habermann, J. Scott, F. Haunert, Cambridge Combinatorial Limited, WO/1999/058475, 1999; S. V. Ley, I. R. Baxendale, R. N. Bream, P. S. Jackson, A. G. Leach, D. A. Longbottom, M. Nesi, J. S. Scott, R. I. Storer, S. J. Taylor, J. Chem. Soc. Perkin Trans. 1 2000, 3815–4195.
- 2I. R. Baxendale, S. V. Ley, Bioorg. Med. Chem. Lett. 2000, 10, 1983–1986; S. V. Ley, I. R. Baxendale, Nat. Rev. Drug Discovery 2002, 1, 573–586; S. V. Ley, I. R. Baxendale, G. Brusotti, M. Caldarelli, A. Massi, M. Nesi, Farmaco 2002, 57, 321–330; S. V. Ley, I. R. Baxendale, Chem. Rec. 2002, 2, 377–388; S. V. Ley, I. R. Baxendale, M. Ernst, W.-R. Krahnert, Synlett 2002, 1641–1644; I. R. Baxendale, G. Brusotti, M. Matsuoka, S. V. Ley, J. Chem. Soc. Perkin Trans. 1 2002, 143–154; I. R. Baxendale, A.-L. Lee, S. V. Ley, Synlett 2002, 516–518; I. R. Baxendale, S. V. Ley, W. Lumeras, M. Nesi, Comb. Chem. High Throughput Screening 2002, 5, 197–199; I. R. Baxendale, S. V. Ley, H. F. Sneddon, Synlett 2002, 775–777; I. R. Baxendale, S. V. Ley, Curr. Org. Chem. 2005, 9, 1521–1534.
- 3I. R. Baxendale, A.-L. Lee, S. V. Ley, Synlett 2001, 1482–1484; I. R. Baxendale, S. V. Ley, M. Nesi, C. Piutti, Tetrahedron 2002, 58, 6285–6304; I. R. Baxendale, A.-L. Lee, S. V. Ley, J. Chem. Soc. Perkin Trans. 1 2002, 1850–1857; I. R. Baxendale, T. D. Davidson, S. V. Ley, R. H. Perni, Heterocycles 2003, 60, 2707–2715; R. I. Storer, T. Takemoto, P. S. Jackson, D. S. Brown, I. R. Baxendale, S. V. Ley, Chem. Eur. J. 2004, 10, 2529–2547; I. R. Baxendale, S. V. Ley, Curr. Org. Chem. 2005, 9, 1521–1534; I. R. Baxendale, S. V. Ley, Ind. Eng. Chem. Res. 2005, 44, 8588–8592.
- 4J. Siu, I. R. Baxendale, S. V. Ley, Org. Biomol. Chem. 2004, 2, 160–167;
S. Saaby, I. R. Baxendale, S. V. Ley, Org. Biomol. Chem. 2005, 3, 3365–3368;
I. R. Baxendale, A.-L. Lee, S. V. Ley in Microwave-Assisted Organic Synthesis (Eds.: ), Blackwell, Oxford, 2005, chap. 6, pp. 133–176;
10.1002/9781444305548.ch6 Google ScholarI. R. Baxendale, M. R. Pitts, Chim. Oggi 2006, 24, 41–45; I. R. Baxendale, C. M. Griffiths-Jones, S. V. Ley, G. K. Tranmer, Chem. Eur. J. 2006, 12, 4407–4417; I. R. Baxendale, J. J. Hayward, S. V. Ley, Comb. Chem. High Throughput Screening 2007, 10, 802–836; C. J. Smith, F. J. Iglesias-Sigüenza, I. R. Baxendale, S. V. Ley, Org. Biomol. Chem. 2007, 5, 2758–2761.
- 5A. Studer, S. Hadida, R. Ferritto, S.-Y. Kim, P. Jeger, P. Wipf, D. P. Curran, Science 1997, 275, 823–826;
D. L. Flynn, J. Z. Crich, R. V. Devraj, S. L. Kockerman, J. J. Parlow, M. S. South, S. Woodward, J. Am. Chem. Soc. 1997, 119, 4874–4881;
D. P. Curran, Angew. Chem. 1998, 110, 1230–1255;
10.1002/(SICI)1521-3757(19980504)110:9<1230::AID-ANGE1230>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1174–1196;10.1002/(SICI)1521-3773(19980518)37:9<1174::AID-ANIE1174>3.0.CO;2-P CAS PubMed Web of Science® Google ScholarT. Bosanac, J. Yang, C. S. Wilcox, Angew. Chem. 2001, 113, 1927–1931;10.1002/1521-3757(20010518)113:10<1927::AID-ANGE1927>3.0.CO;2-# Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1875–1879;10.1002/1521-3773(20010518)40:10<1875::AID-ANIE1875>3.0.CO;2-5 CAS PubMed Web of Science® Google ScholarA. Galante, P. Lhoste, D. Sinou, Tetrahedron Lett. 2001, 42, 5425–5427; S. V. Ley, A. Massi, F. Rodríguez, D. C. Horwell, R. A. Lewthwaite, M. C. Pritchard, A. M. Reid, Angew. Chem. 2001, 113, 1088–1090;10.1002/1521-3757(20010316)113:6<1088::AID-ANGE10880>3.0.CO;2-# Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1053–1055;10.1002/1521-3773(20010316)40:6<1053::AID-ANIE10530>3.0.CO;2-D CAS PubMed Web of Science® Google ScholarA. P. Dodds, C. McGregor-Johnson, Tetrahedron Lett. 2002, 43, 2807–2810; J. Yoshida, K. Itami, Chem. Rev. 2002, 102, 3693–3716; P. Lan, J. A. Porco, Jr., M. S. South, J. J. Parlow, J. Comb. Chem. 2003, 5, 660–669; D. P. Curran, X. Wang, Q. Zhang, J. Org. Chem. 2005, 70, 3716–3719; J. Siu, I. R. Baxendale, R. A. Lewthwaite, S. V. Ley, Org. Biomol. Chem. 2005, 3, 3140–3160; S. Mothana, N. Chahal, S. Vanneste, D. G. Hall, J. Comb. Chem. 2007, 9, 193–196.
- 6Reviews of flow chemistry from our laboratory: I. R. Baxendale, J. J. Hayward, S. V. Ley, G. K. Tranmer, ChemMedChem 2007, 2, 768–788; I. R. Baxendale, J. J. Hayward, S. Lanners, S. V. Ley, C. D. Smith in Microreactors in Organic Synthesis and Catalysis, chap. 4.2 (Ed.: ), Wiley-VCH, Weinheim, 2008, pp. 84–122. Examples of flow chemistry from our laboratory: I. R. Baxendale, S. V. Ley, C. D. Smith, G. K. Tranmer, Chem. Commun. 2006, 4835–4837; I. R. Baxendale, C. M. Griffiths-Jones, S. V. Ley, G. K. Tranmer, Synlett 2006, 427–430; I. R. Baxendale, J. Deeley, C. M. Griffiths-Jones, S. V. Ley, S. Saaby, G. K. Tranmer, Chem. Commun. 2006, 2566–2568; M. Baumann, I. R. Baxendale, S. V. Ley, C. D. Smith, G. K. Tranmer, Org. Lett. 2006, 8, 5231–5234; C. D. Smith, I. R. Baxendale, G. K. Tranmer, M. Baumann, S. C. Smith, R. A. Lewthwaite, S. V. Ley, Org. Biomol. Chem. 2007, 5, 1562–1568; C. H. Hornung, M. R. Mackley, I. R. Baxendale, S. V. Ley, Org. Process Res. Dev. 2007, 11, 399–405; S. V. Ley, M. Baumann, I. R. Baxendale, Synlett 2008, 2111–2114; M. Baumann, I. R. Baxendale, S. V. Ley, N. Nikbin, C. D. Smith, Org. Biomol. Chem. 2008, 6, 1587–1593; M. Baumann, I. R. Baxendale, S. V. Ley, N. Nikbin, C. D. Smith, J. P. Tierney, Org. Biomol. Chem. 2008, 6, 1577–1586; I. R. Baxendale, S. V. Ley, C. D. Smith, L. Tamborini, A.-F. Voica, J. Comb. Chem. 2008, 10, 851–857.
- 7Reviews of flow chemistry from other laboratories: G. Jas, A. Kirschning, Chem. Eur. J. 2003, 9, 5708–5723;
P. Hodge, Curr. Opin. Chem. Biol. 2003, 7, 362–373;
K. Jähnisch, V. Hessel, H. Löwe, M. Baerns, Angew. Chem. 2004, 116, 410–451;
10.1002/ange.200300577 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 406–446; A. Kirschning, W. Solodenko, K. Mennecke, Chem. Eur. J. 2006, 12, 5972–5990; B. Ahmed-Omer, J. C. Brandt, T. Wirth, Org. Biomol. Chem. 2007, 5, 355–359; B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan, D. T. McQuade, Chem. Rev. 2007, 107, 2300–2318; V. T. N. Glasnov, C. O. Kappe, Macromol. Rapid Commun. 2007, 28, 395–410; F. Benito-López, R. J. M. Egberink, D. N. Reinhoudt, W. Verboom, Tetrahedron 2008, 64, 10023–10040. Examples of flow chemistry from other laboratories: A. Kirschning, H. Monenschein, R. Wittenberg, Angew. Chem. 2001, 113, 670–701;10.1002/1521-3757(20010216)113:4<670::AID-ANGE6700>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2001, 40, 650–679;10.1002/1521-3773(20010216)40:4<650::AID-ANIE6500>3.0.CO;2-C CAS PubMed Web of Science® Google ScholarG. Jas, A. Kirschning, Chem. Eur. J. 2003, 9, 5708–5723; D. Bernstein, S. France, J. Wolfer, T. Lectka, Tetrahedron: Asymmetry 2005, 16, 3481–3483; B. Desai, C. O. Kappe, J. Comb. Chem. 2005, 7, 641–643; B. D. A. Hook, W. Dohle, P. R. Hirst, M. Pickworth, M. B. Berry, K. I. Booker-Milburn, J. Org. Chem. 2005, 70, 7558–7564; R. V. Jones, L. Godorhazy, N. Varga, D. Szalay, L. Urge, F. Darvas, J. Comb. Chem. 2006, 8, 110–116; F. Bonfils, I. Cazaux, P. Hodge, C. Caze, Org. Biomol. Chem. 2006, 4, 493–497; C. Wiles, P. Watts, S. J. Haswell, Tetrahedron Lett. 2006, 47, 5261–5264; G. Dräger, C. Kiss, U. Kunz, A. Kirschning, Org. Biomol. Chem. 2007, 5, 3657–3664; H. R. Luckarift, B. S. Ku, J. S. Dordick, J. C. Spain, Biotechnol. Bioeng. 2007, 98, 701–705; M. I. Burguete, A. Cornejo, E. García-Verdugo, M. J. Gil, S. V. Luis, J. A. Mayoral, V. Martínez-Merino, M. Sokolova, J. Org. Chem. 2007, 72, 4344–4350; H. R. Sahoo, J. G. Kralj, K. F. Jensen, Angew. Chem. 2007, 119, 5806–5810; Angew. Chem. Int. Ed. 2007, 46, 5704–5708; H. Usutani, Y. Tomida, A. Nagaki, H. Okamoto, T. Nokami, J.-i. Yoshida, J. Am. Chem. Soc. 2007, 129, 3046–3047; C. M. Griffiths-Jones, M. D. Hopkin, D. Jonsson, S. V. Ley, D. J. Tapolczay, E. Vickerstaffe, M. Ladlow, J. Comb. Chem. 2007, 9, 422–430; A. Kirschning, W. Solodenko, G. Jas, U. Kunz, Synthesis 2007, 583–589; C. Csajági, B. Borcsek, K. Niesz, I. Kovacs, Z. Szekelyhidi, Z. Bajko, L. Urge, F. Darvas, Org. Lett. 2008, 10, 1589–1592; R. Ballini, L. Barboni, L. Castrica, F. Fringuelli, D. Lanari, F. Pizzo, L. Vaccaro, Adv. Synth. Catal. 2008, 350, 1218–1224; J. J. M. van der Linden, P. W. Hilberink, C. M. P. Kronenburg, G. J. Kemperman, Org. Process Res. Dev. 2008, 12, 911–920; J. R. McConnell, J. E. Hitt, E. D. Daugs, T. A. Rey, Org. Process Res. Dev. 2008, 12, 940–945; A. Odedra, K. Geyer, T. Gustafsson, R. Gilmour, P. H. Seeberger, Chem. Commun. 2008, 3025–3027; T. Gustafsson, R. Gilmour, P. H. Seeberger, Chem. Commun. 2008, 3022–3024; T. Gustafsson, F. Pontén, P. H. Seeberger, Chem. Commun. 2008, 1100–1102; D. Grant, R. Dahl, N. D. P. Cosford, J. Org. Chem. 2008, 73, 7219–7223; K. Koch, R. J. F. van den Berg, P. J. Nieuwland, R. Wijtmans, H. E. Schoemaker, J. C. M. van Hest, F. P. J. T. Rutjes, Biotechnol. Bioeng. 2008, 99, 1028–1033; J.-i. Yoshida, A. Nagaki, T. Yamada, Chem. Eur. J. 2008, 14, 7450–7459; I. Ryu, T. Fukuyama, M. T. Rahman, M. Sato, Synlett 2008, 151; D. R. J. Acke, C. V. Stevens, B. I. Roman, Org. Process Res. Dev. 2008, 12, 921–928; K. Tanaka, S. Motomatsu, K. Koyama, K. Fukase, Tetrahedron Lett. 2008, 49, 2010–2012; T. Fukuyama, M. Kobayashi, M. T. Rahman, N. Kamata, I. Ryu, Org. Lett. 2008, 10, 533–536; S. Ceylan, C. Friese, C. Lammel, K. Mazac, A. Kirschning, Angew. Chem. 2008, 120, 9083–9086;10.1002/ange.200801474 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8950–8953.
- 8S. Ohira, Synth. Commun. 1989, 19, 561–564; G. J. Roth, B. Liepold, S. G. Müller, H. J. Bestmann, Synthesis 2004, 59–62; J. Pietruszka, A. Witt, Synthesis 2006, 4266–4268.
- 9Omnifit columns typically 100×10 mm commercially available from Kinesis. Website http://www.kinesis.co.uk/ accessed 29 January 2009.
- 10Vapourtec R2+/R4 commercially available from Vapourtec Ltd, Website http://www.vapourtec.co.uk/ accessed 29 January 2009.
- 11M. Smietana, D. Luvino, C. Amalric, J.-J. Vasseur, Synlett 2007, 3037–3041.
- 12C. Girard, E. Onen, M. Aufort, S. Beauviere, E. Samson, J. Herscovici, Org. Lett. 2006, 8, 1689–1692; C. D. Smith, I. R. Baxendale, S. Lanners, J. J. Hayward, S. C. Smith, S. V. Ley, Org. Biomol. Chem. 2007, 5, 1559–1561.