A Convenient Method for the Synthesis of α-Ethynylphospholes and Modulation of Their π-Conjugated Systems†
Yoshihiro Matano Prof. Dr.
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2571
Search for more papers by this authorMakoto Nakashima
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2571
Search for more papers by this authorHiroshi Imahori Prof. Dr.
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2571
Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan)
Fukui Institute for Fundamental Chemistry, Kyoto University, Sakyo-ku, Kyoto 606-8103 (Japan)
Search for more papers by this authorYoshihiro Matano Prof. Dr.
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2571
Search for more papers by this authorMakoto Nakashima
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2571
Search for more papers by this authorHiroshi Imahori Prof. Dr.
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2571
Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Nishikyo-ku, Kyoto 615-8510 (Japan)
Fukui Institute for Fundamental Chemistry, Kyoto University, Sakyo-ku, Kyoto 606-8103 (Japan)
Search for more papers by this authorThis research was partially supported by a research grant from The Murata Science Foundation.
Graphical Abstract
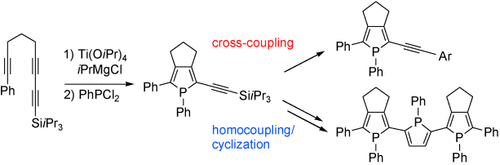
Mind the (narrow) gap: α-Ethynylphospholes generated in situ from the corresponding silyl-capped precursors were converted into a series of α-(arylethynyl) phospholes bearing functional substituents as well as an α,α′-linked terphosphole (see scheme). The terphosphole has a narrow HOMO–LUMO gap owing to efficient π conjugation over the three phosphole rings.
Abstract
Mind the (narrow) gap: α-Ethynylphospholes generated in situ from the corresponding silyl-capped precursors were converted into a series of α-(arylethynyl) phospholes bearing functional substituents as well as an α,α′-linked terphosphole (see scheme). The terphosphole has a narrow HOMO–LUMO gap owing to efficient π conjugation over the three phosphole rings.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200900542_sm_miscellaneous_information.pdf2.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Sonogashira, J. Organomet. Chem. 2002, 653, 46–49;
- 1bR. R. Tykwinski, Angew. Chem. 2003, 115, 1604–1606;
10.1002/ange.200201617 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1566–1568;
- 1cE. Negishi, L. Anastasia, Chem. Rev. 2003, 103, 1979–2018;
- 1dR. Chinchilla, C. Nájera, Chem. Rev. 2007, 107, 874–922.
- 2
- 2aU. H. F. Bunz, Chem. Rev. 2000, 100, 1605–1644;
- 2bJ. M. Tour, Acc. Chem. Res. 2000, 33, 791–804.
- 3For reviews, see:
- 3aF. Mathey, Angew. Chem. 2003, 115, 1616–1643;
10.1002/ange.200200557 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1578–1604;
- 3bM. Hissler, P. W. Dyer, R. Réau, Coord. Chem. Rev. 2003, 244, 1–44;
- 3cL. D. Quin, Curr. Org. Chem. 2006, 10, 43–78;
- 3dT. Baumgartner, R. Réau, Chem. Rev. 2006, 106, 4681–4727 (Correction: T. Baumgartner, R. Réau, Chem. Rev. 2007, 107, 303);
- 3eM. Hissler, C. Lescop, R. Réau, Pure Appl. Chem. 2007, 79, 201–212;
- 3fM. G. Hobbs, T. Baumgartner, Eur. J. Inorg. Chem. 2007, 3611–3628;
- 3gC. Lescop, M. Hissler in Tomorrow’s Chemistry Today (Ed.: ), Wiley-VCH, Weinheim, 2008, pp. 295–319;
10.1002/9783527621767.ch12 Google Scholar
- 3hY. Matano, H. Imahori, Org. Biomol. Chem. 2009, 7, 1258–1271.
- 4For example, see:
- 4aS. S. H. Mao, T. D. Tilley, Macromolecules 1997, 30, 5566–5569;
- 4bC. Hay, M. Hissler, C. Fischmeister, J. Rault-Berthelot, L. Toupet, L. Nyulászi, R. Réau, Chem. Eur. J. 2001, 7, 4222–4236;
10.1002/1521-3765(20011001)7:19<4222::AID-CHEM4222>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 4cY. Morisaki, Y. Aiki, Y. Chujo, Macromolecules 2003, 36, 2594–2597;
- 4dI. Tomita, M. Ueda, Macromol. Symp. 2004, 209, 217–230;
- 4eT. Baumgartner, W. Bergmans, T. Kárpáti, T. Neumann, M. Nieger, L. Nyulázi, Chem. Eur. J. 2005, 11, 4687–4699;
- 4fT. Sanji, K. Shiraishi, M. Tanaka, Org. Lett. 2007, 9, 3611–3614;
- 4gN. H. T. Huy, B. Donnadieu, F. Mathey, A. Muller, K. Colby, C. J. Bardeen, Organometallics 2008, 27, 5521–5524;
- 4hH. Tsuji, K. Sato, L. Ilies, Y. Itoh, Y. Sato, E. Nakamura, Org. Lett. 2008, 10, 2263–2265;
- 4iA. Fukazawa, H. Yamada, S. Yamaguchi, Angew. Chem. 2008, 120, 5664–5667;
10.1002/ange.200801834 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5582–5585.
- 5S. Holand, F. Gandolfo, L. Ricard, F. Mathey, Bull. Soc. Chim. Fr. 1996, 133, 33–37.
- 6
- 6aY. Matano, T. Miyajima, T. Nakabuchi, Y. Matsutani, H. Imahori, J. Org. Chem. 2006, 71, 5792–5795;
- 6bY. Matano, T. Nakabuchi, T. Miyajima, H. Imahori, H. Nakano, Org. Lett. 2006, 8, 5713–5716;
- 6cY. Matano, T. Miyajima, H. Imahori, Y. Kimura, J. Org. Chem. 2007, 72, 6200–6205;
- 6dT. Miyajima, Y. Matano, H. Imahori, Eur. J. Org. Chem. 2008, 255–259;
- 6eY. Matano, T. Miyajima, T. Fukushima, H. Kaji, Y. Kimura, H. Imahori, Chem. Eur. J. 2008, 14, 8102–8115.
- 7F. Sato, H. Urabe, S. Okamoto, Chem. Rev. 2000, 100, 2835–2886.
- 8J. J. Langer, G. P. Moss, Mol. Cryst. Liq. Cryst. 1993, 237, 457–461.
- 911 b: monoclinic, 0.45×0.15×0.05 mm3, P21/c, a=15.072(6), b=7.859(3), c=17.645(6) Å, β=92.546(4)°, V=2088.0(14) Å3, Z=4, ρcalcd=1.391 g cm−3, μ=1.627 cm−1, collected: 15 573, independent: 4724, parameters: 290, Rw=0.0826, R=0.0426 (I>2.00 σ(I)), GOF=1.072. 14: monoclinic, 0.20×0.15×0.08 mm3, P21/n, a=7.887(4), b=25.483(13), c=20.207(11) Å, β=94.141(8)°, V=4051(4) Å3, Z=4, ρcalcd=1.301 g cm−3, μ=3.14 cm−1, collected: 28 319, independent: 9062, parameters: 488, Rw=0.1618, R=0.0859 (I>2.00 σ(I)), GOF=1.060. CCDC 717638 (11 b) and CCDC 717639 (14) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.)
- 10The λab values observed for 10 a–c are red-shifted by 42–55 nm relative to those reported for 2,5-bis(arylethynyl) thiophenes with the same para substituents: J. S. Siddle et al., New J. Chem. 2007, 31, 841–851; see the Supporting Information.
- 11M. J. Kamlet, J.-L. M. Abboud, M. H. Abraham, R. W. Taft, J. Org. Chem. 1983, 48, 2877–2887.
- 12
- 12aJ.-P. Gisselbrecht, N. N. P. Moonen, C. Boudon, M. B. Nielsen, F. Diederich, M. Gross, Eur. J. Org. Chem. 2004, 2959–2972;
- 12bB. Schäfer, E. Öberg, M. Kritikos, S. Ott, Angew. Chem. 2008, 120, 8352–8355;
10.1002/ange.200802253 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8228–8231.
- 13For the synthesis of 2,5-diaryl phospholes from 1,3-diynes and PhPH2, see:
- 13aG. Märkl, R. Potthast, Angew. Chem. 1967, 79, 58;
10.1002/ange.19670790119 Google ScholarAngew. Chem. Int. Ed. Engl. 1967, 6, 86;
- 13bE. H. Braye, I. Caplier, R. Saussez, Tetrahedron 1971, 27, 5523–5537;
- 13cM. Ogasawara, A. Ito, K. Yoshida, T. Hayashi, Organometallics 2006, 25, 2715–2718.
- 14E. Deschamps, L. Ricard, F. Mathey, Angew. Chem. 1994, 106, 1214–1217; Angew. Chem. Int. Ed. Engl. 1994, 33, 1158–1161.
- 15S. A. Lee, S. Hotta, F. Nakanishi, J. Phys. Chem. A 2000, 104, 1827–1833.
- 16ΔEHOMO–LUMO [eV]=1240 {(1/λab+1/λem)/2} [nm−1].
- 17D. Delaere, M. T. Nguyen, L. G. Vanquickenborne, Phys. Chem. Chem. Phys. 2002, 4, 1522–1530: The HOMO–LUMO gaps of unsubstituted terphosphole and terthiophene were calculated to be 2.7 and 3.3 eV, respectively, at the B3LYP/SV(P) level.