A Stair-Shaped Molecular Silver(0) Chain†
Hoi Ri Moon
Department of Chemistry, Seoul National University, Seoul 151-747 (Korea), Fax: (+82) 2-886-8516
Search for more papers by this authorCheol Ho Choi Prof.
Department of Chemistry, Kyungpook National University, Taegu 702-701 (Korea)
Search for more papers by this authorMyunghyun Paik Suh Prof.
Department of Chemistry, Seoul National University, Seoul 151-747 (Korea), Fax: (+82) 2-886-8516
Search for more papers by this authorHoi Ri Moon
Department of Chemistry, Seoul National University, Seoul 151-747 (Korea), Fax: (+82) 2-886-8516
Search for more papers by this authorCheol Ho Choi Prof.
Department of Chemistry, Kyungpook National University, Taegu 702-701 (Korea)
Search for more papers by this authorMyunghyun Paik Suh Prof.
Department of Chemistry, Seoul National University, Seoul 151-747 (Korea), Fax: (+82) 2-886-8516
Search for more papers by this authorThis work was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD Basic Research Promotion Fund, KRF-2005-084-C00020), and by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST, No. R11-2005-008-00000-0).
Graphical Abstract
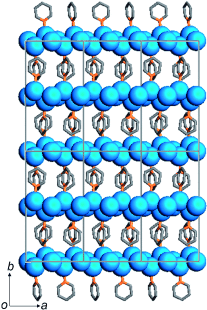
Bound in chains: The molecular Ag0 chain [Ag4py2]n (py=C5H5N) is formed from two covalently linked infinite zigzag chains of Ag, in which AgAg bonds alternate between long and short (see picture; blue Ag, gray C, orange N). Theoretical calculations indicate that partially positively (+0.35) and negatively (−0.30) charged Ag atoms are alternately located along the chain, which has a bandgap of 4.1 eV.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200803465_sm_miscellaneous_information.pdf1.7 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. R. Bermejo, A. M. González-Noya, R. M. Pedrido, M. J. Romero, M. Vázquez, Angew. Chem. 2005, 117, 4254–4259;
10.1002/ange.200500840 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4182–4187;
- 1bM. Shatruk, A. Dragulescu-Andrasi, K. E. Chambers, S. A. Stoian, E. L. Bominaar, C. Achim, K. R. Dunbar, J. Am. Chem. Soc. 2007, 129, 6104–6116.
- 2
- 2aQ.-M. Wang, T. C. W. Mak, Chem. Eur. J. 2003, 9, 44–50;
- 2bQ.-M. Wang, H. K. Lee, T. C. W. Mak, New J. Chem. 2002, 26, 513–515.
- 3
- 3aT. Yamaguchi, F. Yamazaki, T. Ito, J. Am. Chem. Soc. 2001, 123, 743–744;
- 3bJ. F. Berry, F. A. Cotton, L. M. Daniels, C. A. Murillo, J. Am. Chem. Soc. 2002, 124, 3212–3213;
- 3cJ. F. Berry in Multiple Bonds Between Metal Atoms (Eds.: ), Springer Science and Business Media Inc., New York, 2005, pp. 669–706.
10.1007/0-387-25829-9_15 Google Scholar
- 4
- 4aI-W. P. Chen, M.-D. Fu, W.-H. Tseng, J.-Y. Yu, S.-H. Wu, C.-J. Ku, C.-h. Chen, S.-M. Peng, Angew. Chem. 2006, 118, 5946–5950; Angew. Chem. Int. Ed. 2006, 45, 5814–5818;
- 4bS.-Y. Lin, I.-W. P. Chen, C.-h. Chen, M.-H. Hsieh, C.-Y. Yeh, T.-W. Lin, Y.-H. Chen, S.-M. Peng, J. Phys. Chem. B 2004, 108, 959–964.
- 5P. L. Stiles, R. E. Miller, J. Phys. Chem. A 2007, 111, 7382–7390.
- 6
- 6aZ.-M. Tian, Y. Tian, W.-M. Wei, T.-J. He, D.-M. Chen, F.-C. Liu, Chem. Phys. Lett. 2006, 420, 550–555;
- 6bM. Pereiro, D. Baldomir, Phys. Rev. A 2007, 75, 033202;
- 6cG. F. Zhao, Y. Lei, Z. Zeng, Chem. Phys. 2006, 327, 261–268.
- 7F. Bachechi, A. Burini, M. Fontani, R. Galassi, A. Macchioni, B. R. Pietroni, P. Zanello, C. Zuccaccia, Inorg. Chim. Acta 2001, 323, 45–54.
- 8
- 8aH. Yamada, J. Michalik, H. Sadlo, J. Perlinska, S. Takenouchi, S. Shimomura, Y. Uchida, Appl. Clay Sci. 2001, 19, 173–178;
- 8bJ. Michalik, L. Kevan, J. Am. Chem. Soc. 1986, 108, 4247–4253.
- 9
- 9aM. P. Suh, H. R. Moon, E. Y. Lee, S. Y. Jang, J. Am. Chem. Soc. 2006, 128, 4710–4718;
- 9bH. R. Moon, J. H. Kim, M. P. Suh, Angew. Chem. 2005, 117, 1287–1291;
10.1002/ange.200461408 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1261–1265;
- 9cY. E. Cheon, M. P. Suh, Chem. Eur. J. 2008, 14, 3961–3967.
- 10
- 10aM. P. Suh, Y. E. Cheon, E. Y. Lee, Chem. Eur. J. 2007, 13, 4208–4215;
- 10bE. Y. Lee, S. Y. Jang, M. P. Suh, J. Am. Chem. Soc. 2005, 127, 6374–6381;
- 10cE. Y. Lee, M. P. Suh, Angew. Chem. 2004, 116, 2858–2861; Angew. Chem. Int. Ed. 2004, 43, 2798–2801;
- 10dH. J. Choi, M. P. Suh, J. Am. Chem. Soc. 2004, 126, 15844–15851;
- 10eM. P. Suh, J. W. Ko, H. J. Choi, J. Am. Chem. Soc. 2002, 124, 10976–10977;
- 10fK. S. Min, M. P. Suh, J. Am. Chem. Soc. 2000, 122, 6834–6840.
- 11
- 11aZ. Shervani, Y. Ikushima, M. Sato, H. Kawanami, Y. Hakuta, T. Yokoyama, T. Nagase, H. Kuneida, K. Aramaki, Colloid Polym. Sci. 2008, 286, 403–410;
- 11bC. Y. Kim, S. H. Jeong, S.-C. Yi, J. Ceram. Process. Res. 2007, 8, 445–449;
- 11cB. He, J. J. Tan, K. Y. Liew, H. Liu, J. Mol. Catal. A 2004, 221, 121–126;
- 11dL.-J. Chen, C.-C. Wan, Y.-Y. Wang, J. Colloid Interface Sci. 2006, 297, 143–150.
- 12L. M. Engelhardt, S. Gotsis, P. C. Healy, J. D. Kildea, B. W. Skelton, A. H. White, Aust. J. Chem. 1989, 42, 149–176.
- 13We prepared [Ag2I2py2]n according to the method described in Ref. [12] and obtained an X-ray single crystal structure and EDS data. The X-ray crystallographic data are provided in the Supporting Information.
- 14T. R. Cundari, W. J. Stevens, J. Chem. Phys. 1993, 98, 5555–5565.
- 15M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis, J. A. Montgomery, Jr., J. Comput. Chem. 1993, 14, 1347–1363.
- 16M. Kertesz, C. H. Choi, S. Yang, Chem. Rev. 2005, 105, 3448–3481.