Evidence for the Existence of a Terminal Imidoscandium Compound: Intermolecular CH Activation and Complexation Reactions with the Transient ScNAr Species†
Jennifer Scott Dr.
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorFalguni Basuli
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorAlison R. Fout
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorJohn C. Huffman Dr.
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorDaniel J. Mindiola Prof. Dr.
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorJennifer Scott Dr.
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorFalguni Basuli
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorAlison R. Fout
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorJohn C. Huffman Dr.
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorDaniel J. Mindiola Prof. Dr.
Department of Chemistry and the Molecular Structure Center, Indiana University, Bloomington, IN 47405 (USA), Fax: (+1) 812-855-8300
Search for more papers by this authorThe authors thank the NSF (CHE-0348941), the Sloan and the Dreyfus Foundation for financial support of this research. J.S. acknowledges NSERC for a postdoctoral fellowship. Dr. Hongjun Fan is thanked for performing DFT calculations and Dr. John Tomaszewski is also acknowledged for aid with multinuclear NMR spectroscopic studies. Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Graphical Abstract
Don't blink or you'll miss it: A transient imidoscandium complex (see scheme) can be generated by several routes. The imido ligand can activate the CH bond in pyridine and benzene, and it can be complexed with Al(CH3)3 to yield an imide zwitterion. A combination of isotopic labeling, reactivity, and theoretical studies strongly endorse formation of a terminal imido ligand at scandium.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200803325_sm_miscellaneous_information.pdf496.6 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. E. Wigley, Prog. Inorg. Chem. 1994, 42, 239; N. Hazari, P. Mountford, Acc. Chem. Res. 2005, 38, 839; A. L. Odom, Dalton Trans. 2005, 225; A. P. Duncan, R. G. Bergman, Chem. Rec. 2002, 2, 431; A. R. Fout, U. J. Kilgore, D. J. Mindiola, Chem. Eur. J. 2007, 13, 9428; D. J. Mindiola, Acc. Chem. Res. 2006, 39, 813.
- 2
- 2aF. A. A. Trifonov, M. N. Bochkarev, H. Schumann, J. Loebel, Angew. Chem. 1991, 103, 1170; Angew. Chem. Int. Ed. Engl. 1991, 30, 1149;
- 2bN. S. Emelyanova, M. N. Bochkarev, H. Schumann, J. Lebel, L. Esser, Koord. Khim. 1994, 20, 789;
- 2cZ. Xie, S. Wang, Q. Yang, T. C. W. Mak, Organometallics 1999, 18, 1578;
- 2dS. Wang, Q. Yang, T. C. W. Mak, Z. Xie, Organometallics 1999, 18, 5511;
- 2eH. S. Chan, H. W. Li, Z. Xie, Chem. Commun. 2002, 652;
- 2fJ. C. Gordon, G. R. Giesbrecht, D. L. Clark, P. J. Hay, D. W. Keogh, R. Poli, B. L. Scott, J. G. Watkin, Organometallics 2002, 21, 4726;
- 2gA. G. Avent, P. B. Hitchcock, A. V. Khvostov, M. F. Lappert, A. V. Protchenko, Dalton Trans. 2004, 2272.
- 3D. J. Beetstra, A. Meetsma, B. Hessen, J. H. Teuben, Organometallics 2003, 22, 4372.
- 4G. R. Giesbrecht, J. C. Gordon, Dalton Trans. 2004, 2387.
- 5B. C. Bailey, H. Fan, E. W. Baum, J. C. Huffman, M.-H. Baik, D. J. Mindiola, J. Am. Chem. Soc. 2005, 127, 16016; B. C. Bailey, H. Fan, J. C. Huffman, M.-H. Baik, D. Mindiola, J. Am. Chem. Soc. 2007, 129, 8781; B. C. Bailey, A. R. Fout, H. Fan, J. Tomaszewski, J. C. Huffman, D. J. Mindiola, J. Am. Chem. Soc. 2007, 129, 2234; B. C. Bailey, J. C. Huffman, D. J. Mindiola, J. Am. Chem. Soc. 2007, 129, 5302.
- 6J. D. Masuda, K. C. Jantunen, O. V. Ozerov, K. J. T. Noonan, D. P. Gates, B. L. Scott, J. L. Kiplinger, J. Am. Chem. Soc. 2008, 130, 2408.
- 7See the Supporting Information.
- 8M. D. Fryzuk, G. Giesbrecht, S. J. Rettig, Organometallics 1996, 15, 3329.
- 9Crystal data for 2: C38H58ClN2P2Sc, M=685.21, monoclinic, space group P21/c, a=20.6443(16), b=11.3633(9), c=17.3145(13) Å, β=107.585(2)°, V=3872.0(5) Å3, Z=4, ρcalcd=1.175 Mg m−3, T=150(2) K, MoKα: 0.71073 Å, absorption coefficient 0.369 mm−1, F(000)=1472, 22 269 reflections collected, 11 154 independent reflections, Rint=0.0710, GoF=0.810, R=0.0436 [I>2σ(I)], wR2=0.0701 [I>2σ(I)].
- 10Crystal data for 3: C39H61N2P2Sc, M=664.80, monoclinic, space group P21/c, a=20.653(3), b=11.3736(14), c=17.400(2) Å, β=107.674(3)°, V=3894.4(8) Å3, Z=4, ρcalcd=1.134 Mg m−3, T=150(2) K, MoKα: 0.71073 Å, absorption coefficient 0.369 mm−1, F(000)=1440, 69 288 reflections collected, 11 386 independent reflections, Rint=0.0845, GoF=0.901, R=0.0355 [I>2σ(I)], wR2=0.0778 [I>2σ(I)].
- 11B. C. Bailey, H. Fan, J. C. Huffman, M.-H. Baik, D. J. Mindiola, J. Am. Chem. Soc. 2006, 128, 6798.
- 12Crystal data for 4: C45.50H68N3P2Sc, M=763.93, monoclinic, space group P21/c, a=25.0198(9), b=20.7333(8), c=19.0601(7) Å, β=90.2720(10)°, V=9887.2(6) Å3, Z=8, ρcalcd=1.026 Mg m−3, T=150(2) K, MoKα: 0.71073 Å, absorption coefficient 0.243 mm−1, F(000)=3304, 74 624 reflections collected, 19 728 independent reflections, Rint=0.0312, GoF=1.043, R=0.0602 [I>2σ(I)], wR2=0.1923 [I>2σ(I)].
- 13M. E. Thompson, S. M. Baxter, A. R. Bulls, B. J. Burger, M. C. Nolan, B. D. Santarsiero, W. P. Schaefer, J. E. Bercaw, J. Am. Chem. Soc. 1987, 109, 203; W. E. Piers, D. J. H. Emslie, Coord. Chem. Rev. 2002, 233–234, 131, and references therein; R. F. Jordan, Adv. Organomet. Chem. 1991, 32, 325.
- 14Crystal data for 5: C44H73AlN2P2Sc, M=763.92, monoclinic, space group C2/c, a=24.3573(10), b=22.7846(9), c=18.4427(8) Å, β=114.9920(10)°, V=9276.8(7) Å3, Z=8, ρcalcd=1.094 Mg m3−, T=150(2) K, MoKα=0.71073 Å, absorption coefficient 0.276 mm−1, F(000)=3320, 45 314 reflections collected, 9461 independent reflections, Rint=0.0299, GoF=1.025, R=0.0354 [I>2σ(I)], wR2=0.0901 [I>2σ(I)].
- 15B. C. Bailey, A. R. Fout, H. Fan, J. C. Huffman, D. J. Mindiola, Angew. Chem. 2007, 119, 8394; Angew. Chem. Int. Ed. 2007, 46, 8246.
- 16The ScN distance is comparable to the Pauling and Schomaker–Stevenson predicted bond length of 1.923 Å for a double bond using covalent radii with corrections for electronegativities. L. Pauling, The Nature of the Chemical Bond, 3rd ed., Cornell University Press, Ithaca, NY, 1960.
- 17R. R. Schrock, P. R. Sharp, J. Am. Chem. Soc. 1978, 100, 2389.
- 18L. K. Knight, W. E. Piers, R. McDonald, Organometallics 2006, 25, 3289; L. K. Knight, W. E. Piers, P. Fleurat-Lessard, M. Parvez, R. McDonald, Organometallics 2004, 23, 2087.
- 19C. C. Cummins, S. M. Baxter, P. T. Wolczanski, J. Am. Chem. Soc. 1988, 110, 8731; C. C. Cummins, C. P. Schaller, G. D. Van Duyne, P. T. Wolczanski, A. W. E. Chan, R. Hoffmann, J. Am. Chem. Soc. 1991, 113, 2985; C. P. Schaller, P. T. Wolczanski, Inorg. Chem. 1993, 32, 131; J. de With, A. D. Horton, Angew. Chem. 1993, 105, 958; Angew. Chem. Int. Ed. Engl. 1993, 32, 903; P. J. Walsh, F. J. Hollander, R. G. Bergman, Organometallics 1993, 12, 3705; P. Royo, J. Sanchez-Nieves, J. Organomet. Chem. 2000, 597, 61; S. Y. Lee, R. G. Bergman, J. Am. Chem. Soc. 1995, 117, 5877; C. P. Schaller, C. C. Cummins, P. T. Wolczanski, J. Am. Chem. Soc. 1996, 118, 591; J. L. Polse, R. A. Andersen, R. G. Bergman, J. Am. Chem. Soc. 1998, 120, 13405; D. F. Schafer II, P. T. Wolczanski, J. Am. Chem. Soc. 1998, 120, 4881; H. M. Hoyt, M. E. Forrest, R. G. Bergman, J. Am. Chem. Soc. 2004, 126, 1018; J. L. Bennett, P. T. Wolczanski, J. Am. Chem. Soc. 1994, 116, 2179; C. P. Schaller, J. B. Bonanno, P. T. Wolczanski, J. Am. Chem. Soc. 1994, 116, 4133; T. R. Cundari, T. R. Klinckman, P. T. Wolczanski, J. Am. Chem. Soc. 2002, 124, 1481; P. J. Walsh, F. J. Hollander, R. G. Bergman, J. Am. Chem. Soc. 1988, 110, 8729; J. L. Bennett, P. T. Wolczanski, J. Am. Chem. Soc. 1997, 119, 10696; L. M. Slaughter, P. T. Wolczanski, T. R. Klinckman, T. R. Cundari, J. Am. Chem. Soc. 2000, 122, 7953.
- 20Crystal data for 8: C44H63N2P2Sc, M=726.86, triclinic, space group P
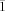 , a=11.7183(6), b=12.2735(6), c=17.2532(8) Å, α=74.9370(10), β=73.8470(10), γ=62.5370(10)°, V=2089.52(18) Å3, Z=2, ρcalcd=1.155 Mg m−3, T=150(2) K, MoKα: 0.71073 Å, absorption coefficient 0.284 mm−1, F(000)=784, 23 174 reflections collected, 7905 independent reflections, Rint=0.0319, GoF=1.013, R=0.0357 [I>2σ(I)], wR2=0.0859 [I>2σ(I)].
, a=11.7183(6), b=12.2735(6), c=17.2532(8) Å, α=74.9370(10), β=73.8470(10), γ=62.5370(10)°, V=2089.52(18) Å3, Z=2, ρcalcd=1.155 Mg m−3, T=150(2) K, MoKα: 0.71073 Å, absorption coefficient 0.284 mm−1, F(000)=784, 23 174 reflections collected, 7905 independent reflections, Rint=0.0319, GoF=1.013, R=0.0357 [I>2σ(I)], wR2=0.0859 [I>2σ(I)].





