Synthesis of Secondary Enamides by Ruthenium-Catalyzed Selective Addition of Amides to Terminal Alkynes†
Lukas J. Gooßen Prof. Dr.
FB Chemie—Organische Chemie, TU Kaiserslautern, Erwin-Schrödinger-Strasse Geb. 54, 67663 Kaiserslautern (Germany), Fax: (+49) 631-205-3921 http://www.chemie.uni-kl.de/goossen
Search for more papers by this authorKifah S. M. Salih
FB Chemie—Organische Chemie, TU Kaiserslautern, Erwin-Schrödinger-Strasse Geb. 54, 67663 Kaiserslautern (Germany), Fax: (+49) 631-205-3921 http://www.chemie.uni-kl.de/goossen
Search for more papers by this authorMathieu Blanchot
FB Chemie—Organische Chemie, TU Kaiserslautern, Erwin-Schrödinger-Strasse Geb. 54, 67663 Kaiserslautern (Germany), Fax: (+49) 631-205-3921 http://www.chemie.uni-kl.de/goossen
Search for more papers by this authorLukas J. Gooßen Prof. Dr.
FB Chemie—Organische Chemie, TU Kaiserslautern, Erwin-Schrödinger-Strasse Geb. 54, 67663 Kaiserslautern (Germany), Fax: (+49) 631-205-3921 http://www.chemie.uni-kl.de/goossen
Search for more papers by this authorKifah S. M. Salih
FB Chemie—Organische Chemie, TU Kaiserslautern, Erwin-Schrödinger-Strasse Geb. 54, 67663 Kaiserslautern (Germany), Fax: (+49) 631-205-3921 http://www.chemie.uni-kl.de/goossen
Search for more papers by this authorMathieu Blanchot
FB Chemie—Organische Chemie, TU Kaiserslautern, Erwin-Schrödinger-Strasse Geb. 54, 67663 Kaiserslautern (Germany), Fax: (+49) 631-205-3921 http://www.chemie.uni-kl.de/goossen
Search for more papers by this authorWe thank the DFG and Umicore AG for financial support.
Graphical Abstract
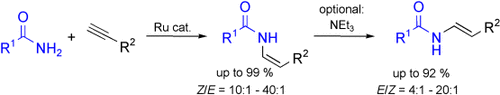
Enamides made easy: A catalyst system generated in situ using bis(2-methallyl)(cycloocta-1,5-diene)ruthenium(II), 1,4-bis(dicyclohexylphosphino)butane, and ytterbium triflate efficiently catalyzes the addition of primary amides to terminal alkynes, selectively forming the Z-anti-Markovnikov enamides. The E isomers are also accessible by combining the hydroamidation with an in situ double-bond isomerization reaction.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200803068_sm_miscellaneous_information.pdf664.5 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Yet, Chem. Rev. 2003, 103, 4283–4306.
- 2
- 2aJ.-H. Lin, Phytochemistry 1989, 28, 621–622;
- 2bI. Stefanuti, S. A. Smith, R. J. K. Taylor, Tetrahedron Lett. 2000, 41, 3735–3738;
- 2cA. Fürstner, C. Brehm, Y. Cancho-Grande, Org. Lett. 2001, 3, 3955–3957.
- 3J. Kohno, Y. Koguchi, M. Nishio, K. Nakao, M. Kuroda, R. Shimizu, T. Ohnuki, S. Komatsubara, J. Org. Chem. 2000, 65, 990–995.
- 4B. Kunze, R. Jansen, G. Höfle, H. Reichenbach, J. Antibiot. 1994, 47, 881–886.
- 5
- 5aS. Ghosh, D. B. Datta, N. Sen, Synth. Commun. 1987, 17, 299–307;
- 5bA. Maxwell, D. Rampersad, J. Nat. Prod. 1989, 52, 411–414;
- 5cJ. C. Estévez, M. C. Villaverde, R. J. Estévez, J. A. Seijas, L. Castedo, Synth. Commun. 1990, 20, 503–507.
- 6For reviews, see:
- 6aD. J. Faulkner, Nat. Prod. Rep. 1999, 16, 155–198;
- 6bD. J. Faulkner, Nat. Prod. Rep. 2000, 17, 7–55.
- 7J. C. Estévez, R. J. Estévez, L. Castedo, Tetrahedron 1995, 51, 10801–10810.
- 8G. J. Roff, R. C. Lloyd, N. J. Turner, J. Am. Chem. Soc. 2004, 126, 4098–4099.
- 9C. E. Willans, C. A. Mulder, J. G. de Vries, H. M. de Vries, J. Organomet. Chem. 2003, 687, 494–497.
- 10
- 10aP. Dupau, P. Le Gendre, C. Bruneau, P. H. Dixneuf, Synlett 1999, 1832–1834;
- 10bX. Wang, J. A. Porco, Jr., J. Org. Chem. 2001, 66, 8215–8221;
- 10cA. Bayer, M. E. Maier, Tetrahedron 2004, 60, 6665–6677.
- 11
- 11aR. K. Boeckman, Jr., S. W. Goldstein, M. A. Walters, J. Am. Chem. Soc. 1988, 110, 8250–8252;
- 11bS. S. Kinderman, J. H. van Maarseveen, H. E. Schoemaker, H. Hiemstra, F. P. J. T. Rutjes, Org. Lett. 2001, 3, 2045–2048.
- 12S. Krompiec, M. Pigulla, N. Kuźnik, M. Krompiec, B. Marciniec, D. Chadyniak, J. Kasperczyk, J. Mol. Catal. A 2005, 225, 91–101.
- 13
- 13aT. Hosokawa, M. Takano, Y. Kuroki, S.-I. Murahashi, Tetrahedron Lett. 1992, 33, 6643–6646;
- 13bJ. M. Lee, D.-S. Ahn, D. Y. Jung, J. Lee, Y. Do, S. K. Kim, S. Chang, J. Am. Chem. Soc. 2006, 128, 12954–12962.
- 14H. Tsujita, Y. Ura, S. Matsuki, K. Wada, T.-A. Mitsudo, T. Kondo, Angew. Chem. 2007, 119, 5252–5255;
10.1002/ange.200701356 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5160–5163.
- 15
- 15aR. Brettle, A. J. Mosedale, J. Chem. Soc. Perkin Trans. 1 1988, 2185–2195;
- 15bK. Kuramochi, H. Watanabe, T. Kitahara, Synlett 2000, 397–399.
- 16
- 16aD. J. Ager, Synthesis 1984, 384–398;
- 16bA. Fürstner, C. Brehm, Y. Cancho-Grande, Org. Lett. 2001, 3, 3955–3957.
- 17
- 17aD. J. Wallace, D. J. Klauber, C.-Y. Chen, R. P. Volante, Org. Lett. 2003, 5, 4749–4752;
- 17bX. Pan, Q. Cai, D. Ma, Org. Lett. 2004, 6, 1809–1812;
- 17cJ. L. Brice, J. E. Meerdink, S. S. Stahl, Org. Lett. 2004, 6, 1845–1848.
- 18Y. Bolshan, R. A. Batey, Angew. Chem. 2008, 120, 2139–2142;
10.1002/ange.200704711 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2109–2112.
- 19
- 19aL. J. Gooßen, J. E. Rauhaus, G. Deng, Angew. Chem. 2005, 117, 4110–4113; Angew. Chem. Int. Ed. 2005, 44, 4042–4045;
- 19bL. J. Gooßen, M. Blanchot, C. Brinkmann, K. Gooßen, R. Karch, A. Rivas-Nass, J. Org. Chem. 2006, 71, 9506–9509.
- 20For related hydroamidation reactions, see:
- 20aT. Kondo, A. Tanaka, S. Kotachi, Y. Watanabe, J. Chem. Soc. Chem. Commun. 1995, 413–414;
- 20bS. Yudha, Y. Kuninobu, K. Takai, Org. Lett. 2007, 9, 5609–5611;
- 20cH. Qian, R. A. Widenhoefer, Org. Lett. 2005, 7, 2635–2638.
- 21For addition reactions of other nucleophiles to alkynes, see:
- 21aT.-A. Mitsudo, Y. Hori, Y. Watanabe, J. Org. Chem. 1985, 50, 1566–1568;
- 21bH. Doucet, N. Derrien, Z. Kabouche, C. Bruneau, P. H. Dixneuf, J. Organomet. Chem. 1997, 551, 151–157;
- 21cL. J. Gooßen, J. Paetzold, D. Koley, Chem. Commun. 2003, 706–707;
- 21dF. Pholki, S. Doye, Chem. Soc. Rev. 2003, 32, 104–114;
- 21eM. Beller, J. Seayad, A. Tillack, H. Jiao, Angew. Chem. 2004, 116, 3448–3479;
10.1002/ange.200300616 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3368–3398;
- 21fM. Tokunaga, T. Suzuki, N. Koga, T. Fukushima, A. Horiuchi, Y. Wakatsuki, J. Am. Chem. Soc. 2001, 123, 11917–11924;
- 21gA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. 2006, 118, 8064–8105;
10.1002/ange.200602454 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7896–7936.
- 22For recent reviews, see:
- 22aF. Alonso, I. P. Beletskaya, M. Yus, Chem. Rev. 2004, 104, 3079–3159;
- 22bC. Bruneau, P. H. Dixneuf, Angew. Chem. 2006, 118, 2232–2260;
10.1002/ange.200501391 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2176–2203.
- 23H. Doucet, B. Martin-Vaca, C. Bruneau, P. H. Dixneuf, J. Org. Chem. 1995, 60, 7247–7255.