Enamide Synthesis by Copper-Catalyzed Cross-Coupling of Amides and Potassium Alkenyltrifluoroborate Salts†
Yuri Bolshan
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-978-5059 http://www.chem.utoronto.ca/staff/RAB/index.html
Search for more papers by this authorRobert A. Batey Prof.
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-978-5059 http://www.chem.utoronto.ca/staff/RAB/index.html
Search for more papers by this authorYuri Bolshan
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-978-5059 http://www.chem.utoronto.ca/staff/RAB/index.html
Search for more papers by this authorRobert A. Batey Prof.
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-978-5059 http://www.chem.utoronto.ca/staff/RAB/index.html
Search for more papers by this authorThe Natural Science and Engineering Research Council (NSERC) of Canada funded this research. Y.B. thanks the NSERC for a postgraduate scholarship (PGS D3) and the Walter C. Sumner Memorial Fellowship for funding. We also thank Dr. Alex Young for mass spectrometry analysis and Dr. Tim Burrow for assistance with NMR spectroscopy.
Graphical Abstract
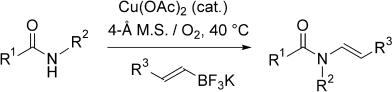
A new partner: Potassium alkenyltrifluoroborate salts undergo coupling with amides to give enamides in the presence of a Cu(OAc)2 catalyst and under mild oxidative conditions (see scheme). The air- and water-stable alkenyltrifluoroborate salts offer a convenient alternative to alkenyl halides as cross-coupling partners. A range of amides undergo coupling including cyclic amides, imides, and carbamates as well as benzamides.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z704711_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see
- 1aL. Yet, Chem. Rev. 2003, 103, 4283–4306;
- 1bM. M. Joullié, D. J. Richard, Chem. Commun. 2004, 2011–2015;
- 1cN.-H. Tan, J. Zhu, Chem. Rev. 2006, 106, 840–895.
- 2For selected references of total syntheses of enamide natural products, see
- 2aA. Fürstner, O. R. Thiel, G. Blanda, Org. Lett. 2000, 2, 3731–3734;
- 2bX. Wang, J. A. Porco, Jr., J. Am. Chem. Soc. 2003, 125, 6040–6041;
- 2cR. Shen, C. T. Lin, E. J. Bowman, B. J. Bowman, J. A. Porco, Jr., J. Am. Chem. Soc. 2003, 125, 7889–7901;
- 2dK. L. Yang, B. Blackman, W. Diederich, P. T. Flaherty, C. J. Mossman, S. Roy, Y. M. Ahn, G. I. Georg, J. Org. Chem. 2003, 68, 10030–10039;
- 2eK. C. Nicolaou, D. W. Kim, R. Baati, A. O'Brate, P. Giannakakou, Chem. Eur. J. 2003, 9, 6177–6191;
- 2fQ. Su, J. S. Panek, J. Am. Chem. Soc. 2004, 126, 2425–8132;
- 2gC. Herb, A. Bayer, M. E. Maier, Chem. Eur. J. 2004, 10, 5649–5660;
- 2hR. Shen, T. Inoue, M. Forgac, J. A. Porco, Jr., J. Org. Chem. 2005, 70, 3686–3692; L. C. Dias, L. G. de Oliveira, J. D. Vilcachagua, F. Nigsch, J. Org. Chem. 2005, 70, 2225–2234;
- 2iP. Cristau, T. Temal-Laïb, M. Bois-Choussy, M.-T. Martin, J.-P. Vors, J. Zhu; C. Sun, J. E. Camp, S. M. Weinreb, Org. Lett. 2006, 8, 1779–1781;
- 2jM. Toumi, F. Couty, G. Evano, Angew. Chem. 2007, 119, 578–581;
10.1002/ange.200602865 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 572–575;
- 2kG. He, J. Wang, D. Ma, Org. Lett. 2007, 9, 1367–1369.
- 3R. L. Larock in Comprehensive Organic Transformations: A Guide to Functional Group Preparations, 2nd ed., Wiley-VCH, New York, 1999, p. 1507.
- 4J. R. Dehli, J. Legros, C. Bolm, Chem. Commun. 2005, 973–986.
- 5Acid-catalyzed condensation of amides and aldehydes:
- 5aC. A. Zezza, M. B. Smith, Synth. Commun. 1987, 17, 729–740;
- 5bM. J. Kiefel, J. Maddock, G. Pattenden, Tetrahedron Lett. 1992, 33, 3227–3230.
- 6Curtius rearrangement of α,β-unsaturated acyl azides: B. B. Snider, F. B. Song, Org. Lett. 2000, 2, 407–408.
- 7Acylation of imines: R. K. Boeckmann, Jr., W. S. Goldstein, M. A. Walters, J. Am. Chem. Soc. 1988, 110, 8250–8252.
- 8Elimination of β-hydroxy-α-silyl amides:
- 8aD. J. Ager, Org. React. 1990, 38, 1–223;
- 8bA. Fürstner, C. Brehm, Y. Cancho-Grande, Org. Lett. 2001, 3, 3955–3957.
- 9Condensation of aldehydes or ketones with nitriles: Z. Rappoport in The Chemistry of Enamines, Wiley, Chichester, 1994, pp. 1441–1533.
10.1002/0470024763 Google Scholar
- 10Ru-catalyzed amidation of alkynes:
- 10aT. Kondo, A. Tanaka, S. Kotachi, Y. Watanabe, J. Chem. Soc. Chem. Commun. 1995, 413–414;
- 10bL. J. Gooßen, J. E. Rauhaus, G. Deng, Angew. Chem. 2005, 117, 4110–4113; Angew. Chem. Int. Ed. 2005, 44, 4042–4045; Ru-catalyzed co-oligomerization of N-vinylamides:
- 10cH. Tsujita, Y. Ura, S. Matsuki, K. Wada, T. Mitsudo, T. Kondo, Angew. Chem. 2007, 119, 5252–5255;
10.1002/ange.200701356 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5160–5163.
- 11Fe- and Rh-catalyzed isomerization of N-allylamides:
- 11aJ. K. Stille, Y. Becker, J. Org. Chem. 1980, 45, 2139–2145;
- 11bS. Sergeyev, M. Hesse, Synlett 2002, 1313–1317;
- 11cS. A. Sergeyev, M. Hesse, Helv. Chim. Acta 2003, 86, 750–755.
- 12Pd-catalyzed intramolecular coupling between amides and alkenyl halides:
- 12aY. Kozawa, M. Mori, Tetrahedron 2002, 58, 111–114; Pd-catalyzed oxidative amidation of conjugated olefins:
- 12bJ. M. Lee, D.-S. Ahn, D. Y. Jung, J. Lee, Y. Do, S. K. Kim, S. Chang, J. Am. Chem. Soc. 2006, 128, 12954–12962; Pd-catalyzed Heck reaction:
- 12cP. Harrison, G. Meek, Tetrahedron Lett. 2004, 45, 9277–9280; Pd-catalyzed coupling between amides and enol ethers:
- 12dA. Klapars, K. R. Campos, C.-y. Chen, R. P. Volante, Org. Lett. 2005, 7, 1185–1188;
- 12eJ. L. Brice, J. E. Meerdink, S. S. Stahl, Org. Lett. 2004, 6, 1845–1848;
- 12fD. J. Wallace, D. J. Klauber, C.-y. Chen, R. P. Volante, Org. Lett. 2003, 5, 4749–4752.
- 13In recent years, there has been an increased interest in copper-catalyzed transformations; for recent reviews, see
- 13aJ. Hassan, M. Sevignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 2002, 102, 1359–1469;
- 13bJ. P. Finet, A. Y. Fedorov, S. Combes, G. Boyer, Curr. Org. Chem. 2002, 6, 597–626;
- 13cK. Kunz, U. Scholz, D. Ganzer, Synlett 2003, 2428–2439;
- 13dS. V. Ley, A. W. Thomas, Angew. Chem. 2003, 115, 5558–5607;
10.1002/ange.200300594 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5400–5449;
- 13eI. P. Beletskaya, A. V. Cheprakov, Coord. Chem. Rev. 2004, 248, 2337–2364;
- 13fS. R. Chemler, P. H. Fuller, Chem. Soc. Rev. 2007, 36, 1153–1160.
- 14T. Ogawa, T. Kiji, K. Hayami, H. Suzuki, Chem. Lett. 1991, 1443–1446.
- 15
- 15aI. Goldberg, Ber. Dtsch. Chem. Ges. 1906, 39, 1691–1692;
- 15bI. Goldberg, Ber. Dtsch. Chem. Ges. 1907, 40, 4541–4546.
- 16
- 16aR. C. Shen, J. A. Porco, Jr., Org. Lett. 2000, 2, 1333–1336;
- 16bR. Shen, C. T. Lin, J. A. Porco, Jr., J. Am. Chem. Soc. 2002, 124, 5650–5651;
- 16cL. Jiang, G. E. Job, A. Klapars, S. L. Buchwald, Org. Lett. 2003, 5, 3667–3669;
- 16dX. Pan, Q. Cai, D. Ma, Org. Lett. 2004, 6, 1809–1812;
- 16eC. Han, R. Shen, S. Su, J. A. Porco, Jr., Org. Lett. 2004, 6, 27–30;
- 16fR. S. Coleman, P.-H. Liu, Org. Lett. 2004, 6, 577–580;
- 16gL. Shen, R. P. Hsung, Y. Zhang, J. E. Antoline, X. Zhang, Org. Lett. 2005, 7, 3081–3084;
- 16hT. Hu, C. Li, Org. Lett. 2005, 7, 2035–2038;
- 16iC. Sun, J. E. Camp, S. M. Weinreb, Org. Lett. 2006, 8, 1779–1781.
- 17P. Y. S. Lam, G. Vincent, D. Bonne, C. G. Clark, Tetrahedron Lett. 2003, 44, 4927–4931.
- 18D. M. T. Chan, P. Y. S. Lam in Boronic Acids (Ed.: ), Wiley-VCH, Weinheim, 2005, pp. 205–240.
- 19For selected references, see
- 19aD. M. T. Chan, K. L. Monaco, R.-P. Wang, M. P. Winters, Tetrahedron Lett. 1998, 39, 2933–2936;
- 19bP. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan, A. Combs, Tetrahedron Lett. 1998, 39, 2941–2944;
- 19cP. Y. S. Lam, G. Vincent, C. G. Clark, S. Deudon, P. K. Jadhav, Tetrahedron Lett. 2001, 42, 3415–3418.
- 20T. D. Quach, R. A. Batey, Org. Lett. 2003, 5, 1381–1384.
- 21T. D. Quach, R. A. Batey, Org. Lett. 2003, 5, 4397–4400.
- 22E. Vedejs, S. C. Fields, M. R. Schrimpf, J. Am. Chem. Soc. 1993, 115, 11612–11613.
- 23H. A. Stefani, R. Cella, A. S. Vieira, Tetrahedron 2007, 63, 3623–3658.
- 24For examples of Suzuki–Miyaura cross-coupling, see
- 24aJ.-P. Genêt, S. Darses, J.-L. Brayer, J.-P. Demoute, Tetrahedron Lett. 1997, 38, 4393–4396;
- 24bG. A. Molander, M. R. Rivero, Org. Lett. 2002, 4, 107–109;
- 24cR. A. Batey, T. D. Quach, Tetrahedron Lett. 2002, 43, 9099–9103;
- 24dG. A. Molander, N. Ellis, Acc. Chem. Res. 2007, 40, 275–286.
- 25For examples of leading references for Rh- or Pd-catalyzed additions to aldehydes, ketones, and enones, see
- 25aR. A. Batey, A. N. Thadani, D. V. Smil, Org. Lett. 1999, 1, 1683–1686;
- 25bM. Pucheault, S. Darses, J.-P. Genêt, Eur. J. Org. Chem. 2002, 3552–3557;
- 25cA. Duursma, J.-G. Boiteau, L. Lefort, J. A. F. Boogers, A. H. M. de Vries, J. G. de Vries, A. J. Minnaard, B. L. Feringa, J. Org. Chem. 2004, 69, 8045–8052;
- 25dM. Pucheault, S. Darses, J.-P. Genêt, J. Am. Chem. Soc. 2004, 126, 15356–15357.
- 26R. A. Altman, S. L. Buchwald, Org. Lett. 2007, 9, 643–646.