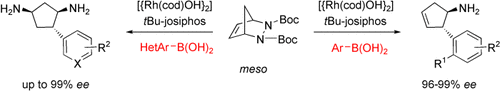Chemodivergence in Enantioselective Desymmetrization of Diazabicycles: Ring-Opening versus Reductive Arylation†
Frederic Menard
Davenport Chemistry Laboratories, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-946-8185
Search for more papers by this authorMark Lautens Prof.
Davenport Chemistry Laboratories, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-946-8185
Search for more papers by this authorFrederic Menard
Davenport Chemistry Laboratories, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-946-8185
Search for more papers by this authorMark Lautens Prof.
Davenport Chemistry Laboratories, University of Toronto, 80 St. George Street, Toronto, ON, M5S 3H6, Canada, Fax: (+1) 416-946-8185
Search for more papers by this authorThis work was supported by NSERC, Merck-Frosst, and the University of Toronto. F.M. thanks the Government of Ontario for a postgraduate scholarship. Solvias Inc, Takasago, and Digital Specialty Chemicals are thanked for generous gifts of chiral ligands. We acknowledge A. Martins for the preparation of some substrates, a referee for useful comments, as well as Y. Bolshan for checking our experimental procedure.
Graphical Abstract
Divergent bicycle paths: A chemodivergent desymmetrization occurs after an initial enantioselective carbometalation step. The reaction brings a solution to the challenging problem of the enantioselective ring-opening of diazabicyclo[2.2.1]heptanes to obtain arylated cyclopentenamines (see scheme, right). An alternative reaction pathway was discovered in which CH insertion/1,4-metal migration occurs to give reductive arylation products (left).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z704708_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. A. Cortez, R. R. Schrock, A. H. Hoveyda, Angew. Chem. 2007, 119, 4618; Angew. Chem. Int. Ed. 2007, 46, 4534;
- 1bM. J. Cook, T. Rovis, J. Am. Chem. Soc. 2007, 129, 9302;
- 1cJ. B. Johnson, E. A. Bercot, C. M. Williams, T. Rovis, Angew. Chem. 2007, 119, 4598;
10.1002/ange.200700816 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4514;
- 1dK. Arai, S. Lucarini, M. M. Salter, K. Ohta, Y. Yamashita, S. Kobayashi, J. Am. Chem. Soc. 2007, 129, 8103;
- 1eY.-H. Cho, V. Zunic, H. Senboku, M. Olsen, M. Lautens, J. Am. Chem. Soc. 2006, 128, 6837;
- 1fJ. M. Berlin, S. D. Goldberg, R. H. Grubbs, Angew. Chem. 2006, 118, 7753;
10.1002/ange.200602469 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 7591;
- 1gC. Bournaud, C. Falciola, T. Lecourt, S. Rosset, A. Alexakis, L. Micouin, Org. Lett. 2006, 8, 3581;
- 1hS. Cabrera, R. G. Arrayas, I. Alonso, J. C. Carretero, J. Am. Chem. Soc. 2005, 127, 17938.
- 2For reviews, see:
- 2aT. Rovis in New Frontiers in Asymmetric Catalysis (Eds.: ), Wiley, New Jersey, 2007, pp. 275–311;
10.1002/9780470098004.ch10 Google Scholar
- 2bM. Pineschi, Eur. J. Org. Chem. 2006, 4979.
- 3For a short review on 1,4-metal migrations, see: S. Ma, Z. Gu, Angew. Chem. 2005, 117, 7680; Angew. Chem. Int. Ed. 2005, 44, 7512. While writing this manuscript, another 1,4-Rh migration example was reported: T. Matsuda, M. Shigeno, M. Murakami, J. Am. Chem. Soc. 2007, DOI: .
- 4
- 4aA related reductive arylation was observed in the ring-opening of oxa- and azanorbornenes, but separation of the products was not possible: C. J. Dockendorff, PhD thesis, University of Toronto (Canada), 2006;
- 4bFor an asymmetric hydroarylation of allenes with boronic acids, see: T. Nishimura, S. Hirabayashi, Y. Yasuhara, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 2556;
- 4cFor asymmetric Pd-mediated hydroarylations of norbornene, see: X.-Y. Wu, H.-D. Xu, Q.-L. Zhou, A. S. C. Chan, Tetrahedron: Asymmetry 2000, 11, 1255.
- 5Racemic Pd-catalyzed variant: J. John, V. S. Sajisha, S. Mohanlal, K. V. Radhakrishnan, Chem. Commun. 2006, 3510.
- 6M. Pineschi, F. Del Moro, P. Crotti, F. Macchia, Org. Lett. 2005, 7, 3605.
- 7F. Bertolini, F. Macchia, M. Pineschi, Tetrahedron Lett. 2006, 47, 9173.
- 8M.-L. Yao, G. Adiwidjaja, D. E. Kaufmann, Angew. Chem. 2002, 114, 3523;
Angew. Chem. Int. Ed. 2002, 41, 3375.
10.1002/1521-3773(20020916)41:18<3375::AID-ANIE3375>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 9In previous work we observed that organoboron reagents add to the alkene of diazabicycle 1 without ring opening:
- 9aM. Lautens, J. J. Mancuso, J. Org. Chem. 2004, 69, 3478;
- 9bN.-W. Tseng, J. J. Mancuso, M. Lautens, J. Am. Chem. Soc. 2006, 128, 5338.
- 10P. E. Finke et al., (Merck),WO 9714671, 1997; see the Supporting Information.
- 11G. DeStevens, P. W. Strachan, M. Dughi, A. Halamandaris, J. Med. Chem. 1961, 3, 533.
- 12K. Ohno, A. Ohtake, S. Nishio, K. Hoshi, S. Tsukamoto, (Toray Industries Inc.), WO 9406785, PCT Int. Appl., 1994, p. 219.
- 13
- 13aF. Menard, T. M. Chapman, C. Dockendorff, M. Lautens, Org. Lett. 2006, 8, 4569;
- 13bFor related work see: T. Miura, Y. Takahashi, M. Murakami, Chem. Commun. 2007, 595.
- 14
- 14aH. A. McManus, M. J. Fleming, M. Lautens, Angew. Chem. 2007, 119, 437; Angew. Chem. Int. Ed. 2007, 46, 433;
- 14bM. Lautens, K. Fagnou, Proc. Natl. Acad. Sci. USA 2004, 101, 5455;
- 14cM. Lautens, C. Dockendorff, Org. Lett. 2003, 5, 3695.
- 15O. Diels, J. H. Bolm, W. Knoll, Justus Liebigs Ann. Chem. 1925, 443, 242.
- 16J. Wu, A. S. C. Chan, Acc. Chem. Res. 2006, 39, 711.
- 17H.-U. Blaser, W. Brieden, B. Pugin, F. Spindler, M. Studer, A. Togni, Top. Catal. 2002, 19, 3.
- 18CCDC 661282 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre at www.ccdc. cam.ac.uk/data request/cif.
- 19Compund 1 c is a free-flowing powder, making for easy handling. Its preparation on scale is also the most convenient one, involving simple filtration of the reaction mixture to obtain analytically pure material.
- 20See the Supporting Information for full experimental details. Strictly anhydrous conditions are not appropriate for this reaction and we thank the referee for this finding.
- 21
- 21aV. H. Lillelund, H. H. Jensen, X. Liang, M. Bols, Chem. Rev. 2002, 102, 515;
- 21bA. Berecibar, C. Grandjean, A. Siriwardena, Chem. Rev. 1999, 99, 779.
- 22C. Bournaud, D. Robic, M. Bonin, L. Micouin, J. Org. Chem. 2005, 70, 3316. See also reference [1g].
- 23
- 23aT. B. Poulsen, C. Alemparte, K. A. Jørgensen, J. Am. Chem. Soc. 2005, 127, 11614;
- 23bH. Ding, G. K. Friestad, Org. Lett. 2004, 6, 637;
- 23cM. Marigo, K. Juhl, K. A. Jørgensen, Angew. Chem. 2003, 115, 1405;
10.1002/ange.200390322 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1367;
- 23dD. A. Evans, T. C. Britton, R. L. Dorow, J. F. Dellaria, Tetrahedron 1988, 44, 5525;
- 23eJ. M. Mellor, N. M. Smith, J. Chem. Soc. Perkin Trans. 1 1984, 2927.
- 24The 1,2,4- and 1,2,5-aromatic substitution patterns occurred in 51 out of 128 drug candidate molecules surveyed, see: J. S. Carey, D. Laffan, C. Thomson, M. T. Williams, Org. Biomol. Chem. 2006, 4, 2337.
- 25The optical rotation for (1R,2S)-21 (62 % ee): [α]
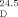 =−36.5 (c=1.25, CHCl3) and [α]
=−36.5 (c=1.25, CHCl3) and [α]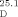 =−39.5 (c=1.00, MeOH); reported for (1S,2R)-21⋅HCl (93 % ee): [α]
=−39.5 (c=1.00, MeOH); reported for (1S,2R)-21⋅HCl (93 % ee): [α]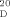 =+56.6 (c=0.77, MeOH)
=+56.6 (c=0.77, MeOH)
- 25aA. Dahnz, G. Helmchen, Synlett 2006, 697;
- 25bA. Dahnz, PhD Thesis, Universität Heidelberg (Germany), 2007.