Total Synthesis of (−)-Reidispongiolide A, an Actin-Targeting Marine Macrolide†
Ian Paterson Prof. Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorKate Ashton Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorRobert Britton Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorGiuseppe Cecere
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorGaëlle Chouraqui Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorGordon J. Florence Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorJonathan Stafford
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorIan Paterson Prof. Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorKate Ashton Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorRobert Britton Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorGiuseppe Cecere
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorGaëlle Chouraqui Dr.
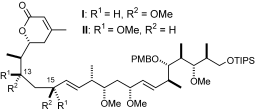
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorGordon J. Florence Dr.
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
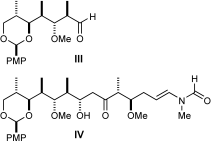
Search for more papers by this authorJonathan Stafford
University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK, Fax: (+44) 1223-336-362 http://www-paterson.ch.cam.ac.uk/
Search for more papers by this authorThis work was supported by the EPSRC (GR/S19929/01), Emmanuel College, Cambridge (Fellowship to G.J.F.), NSERC-Canada (Fellowship to R.B.), Merck Research Laboratories (Scholarship to G.C.), and AstraZeneca. We thank Professor M. V. D'Auria (University of Naples “Federico II”) for providing an authentic sample of natural redispongiolide A and helpful discussions throughout, and Dr. Ray Finlay (AstraZeneca) for assistance.
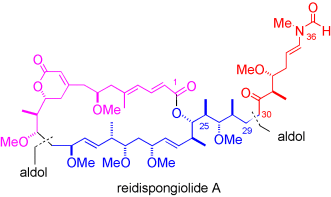
Graphical Abstract
Actin class: A stereoselective synthesis of the microfilament-destabilizing cytotoxic macrolide (−)-reidispongiolide A, isolated from the marine sponge Reidispongia coerulea, uses a convergent aldol-based strategy to construct the 26-membered macrolactone, followed by a coupling with an N-vinylformamide subunit. This constitutes the first synthesis of any member of the reidispongiolide/sphinxolide family.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z702178_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. V. D'Auria, L. Gomez-Paloma, L. Minale, A. Zampella, J.-F. Verbist, C. Roussakis, C. Debitus, J. Patissou, Tetrahedron 1994, 50, 4829.
- 2“The name sphinxolide, from the mysterious Egyptian Sphinx, reflects our difficulties in defining the source and, for some time, the structure of the compound”, F. Pietra (1989); see:
- 2aG. Guella, I. Mancini, G. Chiasera, F. Pietra, Helv. Chim. Acta 1989, 72, 237;
- 2bM. V. D'Auria, L. Gomez-Paloma, L. Minale, A. Zampella, J.-F. Verbist, C. Roussakis, C. Debitus, Tetrahedron 1993, 49, 8657;
- 2cS. Carbonelli, A. Zampella, A, Randazzo, C. Debitus, L. Gomez-Paloma, Tetrahedron 1999, 55, 14665;
- 2dC. Bassarello, G. Bifulco, A. Zampella, M. V. D'Auria, R. Riccio, L. Gomez-Paloma, Eur. J. Org. Chem. 2001, 39.
- 3For reviews on marine macrolide synthesis, see:
- 3aI. Paterson, K.-S. Yeung, Chem. Rev. 2005, 105, 4237;
- 3bR. D. Norcross, I. Paterson, Chem. Rev. 1995, 95, 2041.
- 4K.-S. Yeung, I. Paterson, Angew. Chem. 2002, 114, 4826; Angew. Chem. Int. Ed. 2002, 41, 4632 .
- 5J. S. Allingham, V. A. Klenchin, I. Rayment, Cell. Mol. Life Sci. 2006, 63, 2119.
- 6A. Zampella, C. Bassarello, G. Bifulco, L. Gomez-Paloma, M. V. D'Auria, Eur. J. Org. Chem. 2002, 785.
- 7For other synthetic studies, see:
- 7aI. Paterson, K. Ashton, R. Britton, H. Knust, Org. Lett. 2003, 5, 1963;
- 7bA. Zampella, V. Sepe, R. D'Orsi, G. Bifulco, C. Bassarello, M. V. D'Auria, Tetrahedron: Asymmetry 2003, 14, 1787;
- 7cA. Zampella, V. Sepe, R. D'Orsi, M. V. D'Auria, Lett. Org. Chem. 2004, 1, 308.
- 8I. Paterson, R. Britton, K. Ashton, H. Knust, J. Stafford, Proc. Natl. Acad. Sci. USA 2004, 101, 11986; an alternative stereostructure having the inverted configuration in the C7–C15 region could not be ruled out at this point.
- 9J. S. Allingham, A. Zampella, M. V. D'Auria, I. Rayment, Proc. Natl. Acad. Sci. USA 2005, 102, 14527.
- 10The ketones 9 (82 %) and 12 (84 %) were prepared in 3 steps from methyl (S)-2-methyl-3-hydroxypropionate in a similar manner to that reported previously (Ref. [7a]), see:
- 10aI. Paterson, G. J. Florence, K. Gerlach, J. P. Scott, N. Sereinig, J. Am. Chem. Soc. 2001, 123, 9535;
- 10bI. Paterson, I. M. Donghi, K. Gerlach, Angew. Chem. 2000, 112, 3453;
Angew. Chem. Int. Ed. 2000, 39, 3315.
10.1002/1521-3773(20000915)39:18<3315::AID-ANIE3315>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 11M. A. Blanchette, W. Choy, J. T. Davis, A. P. Essenfeld, S. Masamune, W. R. Roush, T. Sakai, Tetrahedron Lett. 1984, 25, 2183.
- 12
- 12aI. Paterson, J. M. Goodman, M. Isaka, Tetrahedron Lett. 1989, 30, 7121;
- 12bI. Paterson, J. M. Goodman, M. A. Lister, R. C. Schumann, C. K. McClure, R. D. Norcross, Tetrahedron 1990, 46, 4663.
- 13I. Paterson, M. V. Perkins, Tetrahedron 1996, 52, 1811.
- 14
- 14aS. P. Romeril, V. Lee, J. E. Baldwin, T. D. W. Claridge, B. Odell, Tetrahedron Lett. 2003, 44, 7757;
- 14bS. E. Schaus, B. D. Brandes, J. F. Larrow, M. Tokunaga, K. B. Hansen, A. E. Gould, M. E. Furrow, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 1307.
- 15
- 15aE. Negishi, D. E. Van Horn, T. Yoshida, J. Am. Chem. Soc. 1985, 107, 6639;
- 15bC. L. Rand, D. E. Van Horn, M. W. Moore, E. Negishi, J. Org. Chem. 1981, 46, 4093.
- 16
- 16aI. Paterson, K. R. Gibson, R. M. Oballa, Tetrahedron Lett. 1996, 37, 8585;
- 16bI. Paterson, M. J. Coster, D. Y.-K. Chen, K. R. Gibson, D. J. Wallace, Org. Biomol. Chem. 2005, 3, 2410;
- 16cD. A. Evans, P. J. Coleman, B. Côté, J. Org. Chem. 1997, 62, 788.
- 17I. Paterson, K.-S. Yeung, J. B. Smaill, Synlett 1993, 774.
- 18A. DeMico, R. Margarita, L. Parlanti, A. Vescovi, G. Piancatelli, J. Org. Chem. 1997, 62, 6974.
- 19
- 19aI. Paterson, D. J. Wallace, S. M. Velazquez, Tetrahedron Lett. 1994, 35, 9083;
- 19bI. Paterson, D. J. Wallace, Tetrahedron Lett. 1994, 35, 9087;
- 19cI. Paterson, D. J. Wallace, C. J. Cowden, Synthesis 1998, 639.
- 20M. T. Crimmins, S. T. Kirincich, A. J. Wells, A. L. Choy, Synth. Commun. 1998, 28, 3675.
- 21I. Paterson, C. Cowden, C. Watson, Synlett 1996, 209.
- 22T. Mukaiyama, K. Banno, K. Narasaka, J. Am. Chem. Soc. 1974, 96, 7503.
- 23D. A. Evans, K. T. Chapman, E. M. Carreira, J. Am. Chem. Soc. 1988, 110, 3560.
- 24The stereochemical assignment of 33 and its minor 13,15-epi diastereomer was aided by the related preparation of truncated compounds I and II, and their 1H NMR comparison with a fragment library as described in Ref. [8].
- 25J. K. Stille, B. L. Groh, J. Am. Chem. Soc. 1987, 109, 813.
- 26J. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989.
- 27The configuration of the temporarily installed stereocenter at C29 in 38 was assigned by analogy with the corresponding boron aldol reaction of ketone 3 with aldehyde III (2 steps from 8) that generated adduct IV (>20:1 d.r.), in which the configuration of the resulting center that bears a hydroxy group was determined by Mosher ester analysis.
- 28E. M. Burgess, H. R. Penton, E. A. Taylor, J. Org. Chem. 1973, 38, 26.
- 29W. S. Mahoney, D. M. Brestensky, J. M. Stryker, J. Am. Chem. Soc. 1988, 110, 291.
- 30Copies of NMR, CD spectra, and HPLC chromatograms for the synthetic and natural reidispongiolide A, along with comparison tables of 1H and 13C NMR data, are provided in the Supporting Information.