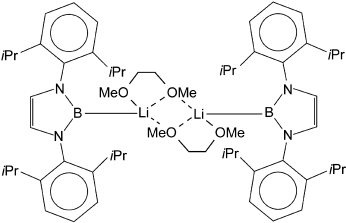
Highlight
Lithiumboryl—A Synthon for a Nucleophilic Boryl Anion
Holger Braunschweig Prof. Dr.,
Holger Braunschweig Prof. Dr.
Institut für Anorganische Chemie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4623
Search for more papers by this authorHolger Braunschweig Prof. Dr.,
Holger Braunschweig Prof. Dr.
Institut für Anorganische Chemie, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany, Fax: (+49) 931-888-4623
Search for more papers by this authorGraphical Abstract
References
- 1H. C. Brown, B. C. Subba Rao, J. Am. Chem. Soc. 1956, 78, 2582–2588.
- 2D. E. Kaufmann, D. S. Matteson, Science of Synthesis, Boron Compounds, Georg Thieme, Stuttgart, 2005.
- 3H. C. Brown, Angew. Chem. 1980, 92, 675–683.
- 4A. Suzuki, N. Miyaura, Chem. Rev. 1995, 95, 2457–2483.
- 5H. C. Brown, B. Singaram, Pure Appl. Chem. 1987, 59, 879–894.
- 6H. C. Brown, A. Pelter, K. Smith, Borane Reagents, Academic Press, New York, 1988.
- 7K. Burgess, M. J. Ohlmeyer, Chem. Rev. 1991, 91, 1179–1191.
- 8I. Beletskaya, A. Pelter, Tetrahedron 1997, 53, 4957–5026.
- 9M. R. Smith III, Prog. Inorg. Chem. 1999, 48, 505–567.
- 10T. B. Marder, N. C. Norman, Top. Catal. 1998, 5, 63–73.
- 11H. Braunschweig, Angew. Chem. 1998, 110, 1882–1898;
10.1002/(SICI)1521-3757(19980703)110:13/14<1882::AID-ANGE1882>3.0.CO;2-5 Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1786–1801.10.1002/(SICI)1521-3773(19980803)37:13/14<1786::AID-ANIE1786>3.0.CO;2-C CAS Web of Science® Google Scholar
- 12G. I. Irvine, M. J. G. Lesley, T. B. Marder, N. C. Norman, C. R. Rice, E. G. Robins, W. R. Roper, G. R. Whittell, L. J. Wright, Chem. Rev. 1998, 98, 2685–2722.
- 13H. Braunschweig, M. Colling, Coord. Chem. Rev. 2001, 223, 1–51.
- 14S. Aldridge, D. L. Coombs, Coord. Chem. Rev. 2004, 248, 535–559.
- 15H. Braunschweig, C. Kollann, D. Rais, Angew. Chem. 2006, 118, 5380–5400; Angew. Chem. Int. Ed. 2006, 45, 5254–5274.
- 16K. T. Giju, F. M. Bickelhaupt, G. Frenking, Inorg. Chem. 2000, 39, 4776–4785.
- 17A. A. Dickinson, D. J. Willock, R. J. Calder, S. Aldridge, Organometallics 2002, 21, 1146–1157.
- 18X. He, J. F. Hartwig, Organometallics 1996, 15, 400–407.
- 19S. Aldridge, D. L. Kays (née Coombs), A. Al-Fawaz, K. M. Jones, P. N. Horton, M. B. Hursthouse, R. W. Harrington, W. Clegg, Chem. Commun. 2006, 2578–2580.
- 20D. Männig, H. Nöth, Angew. Chem. 1985, 97, 854–855;
10.1002/ange.19850971009 Google ScholarAngew. Chem. Int. Ed. Engl. 1985, 24, 878–879.
- 21D. Entwistle, T. B. Marder, Angew. Chem. 2002, 114, 3051–3056;
10.1002/1521-3757(20020816)114:16<3051::AID-ANGE3051>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2927–2931.10.1002/1521-3773(20020816)41:16<2927::AID-ANIE2927>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 22F. Jäkle, J. Inorg. Organomet. Polym. Mater. 2005, 15, 293–307.
- 23H. Braunschweig, D. Rais, Angew. Chem. 2005, 117, 8036–8038; Angew. Chem. Int. Ed. 2005, 44, 7826–7828.
- 24R. W. Auten, C. A. Kraus, J. Am. Chem. Soc. 1952, 74, 3398–3401.
- 25R. Köster, G. Benedikt, Angew. Chem. 1963, 75, 346; Angew. Chem. Int. Ed. Engl. 1963, 2, 219.
- 26R. Köster, G. Benedikt, Angew. Chem. 1964, 76, 650; Angew. Chem. Int. Ed. Engl. 1964, 3, 515.
- 27M. Wagner, N. J. R. van Eikema Hommes, H. Nöth, P. von R. Schleyer, Inorg. Chem. 1995, 34, 607–614.
- 28A. Sundermann, M. Reiher, W. W. Scholler, Eur. J. Inorg. Chem. 1998, 305–310.
10.1002/(SICI)1099-0682(199803)1998:3<305::AID-EJIC305>3.0.CO;2-0 CAS Web of Science® Google Scholar
- 29N. Metzler-Nolte, New J. Chem. 1998, 22, 793–795.
- 30R. J. Brotherton, A. L. McCloskey, L. L. Petterson, H. Steinberg, J. Am. Chem. Soc. 1960, 82, 6242–6245.
- 31T. B. Marder, Science 2006, 314, 69–70.
- 32M. Unverzagt, G. Subramanian, M. Hofmann, P. von R. Schleyer, S. Berger, K. Harms, W. Massa, A. Berndt, Angew. Chem. 1997, 109, 1567–1569;
10.1002/ange.19971091334 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1469–1472.
- 33D. Scheschkewitz, A. Ghaffari, P. Amseis, M. Unverzagt, G. Subramanian, M. Hofmann, P. von R. Schleyer, H. F. Schaefer III, G. Geiseler, W. Massa, A. Berndt, Angew. Chem. 2000, 112, 1329–1332;
10.1002/(SICI)1521-3757(20000403)112:7<1329::AID-ANGE1329>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1272–1275.10.1002/(SICI)1521-3773(20000403)39:7<1272::AID-ANIE1272>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 34For a review on 1,3,2-diazaboroles, see: L. Weber, Coord. Chem. Rev. 2001, 215, 39–77.
- 35Y. Segawa, M. Yamashita, K. Nozaki, Science 2006, 314, 113–115.
- 36A. J. Arduengo III, R. L. Harlow, M. Kline, J. Am. Chem. Soc. 1991, 113, 361–363.
- 37M. Denk, R. Lennon, R. Hayashi, R. West, A. V. Belyakov, H. P. Verne, A. Haaland, M. Wagner, N. Metzler, J. Am. Chem. Soc. 1994, 116, 2691–2692.
- 38W. A. Herrmann, M. Denk, J. Behm, W. Scherer, F.-R. Klingan, H. Bock, B. Solouki, M. Wagner, Angew. Chem. 1992, 104, 1489–1492; Angew. Chem. Int. Ed. Engl. 1992, 31, 1485–1488.
- 39E. S. Schmidt, A. Jockisch, H. Schmidbaur, J. Am. Chem. Soc. 1999, 121, 9758–9759.
- 40W. A. Herrmann, Angew. Chem. 2002, 114, 1342–1363;
Angew. Chem. Int. Ed. 2002, 41, 1290–1309.
10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 41J. Zhu, Z. Lin, T. B. Marder, Inorg. Chem. 2005, 44, 9384–9390.
- 42H. Braunschweig, K. Radacki, D. Rais, D. Scheschkewitz, Angew. Chem. 2005, 117, 5796–5799; Angew. Chem. Int. Ed. 2005, 44, 5651–5654.