Cationic Terminal Aminoborylene Complexes: Controlled Stepwise Insertion into MB and BN Double Bonds†
Glesni A. Pierce
Department of Inorganic Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK, Fax: (+44) 186-527-2690 http://www.chem.ox.ac.uk/researchguide/saldridge.html
Search for more papers by this authorSimon Aldridge Dr.
Department of Inorganic Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK, Fax: (+44) 186-527-2690 http://www.chem.ox.ac.uk/researchguide/saldridge.html
Search for more papers by this authorCameron Jones Prof.
School of Chemistry, Monash University, PO Box 23, Victoria 3800, Australia
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorTimo Gans-Eichler Dr.
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorAndreas Stasch Dr.
School of Chemistry, Monash University, PO Box 23, Victoria 3800, Australia
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorNatalie D. Coombs
Department of Inorganic Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK, Fax: (+44) 186-527-2690 http://www.chem.ox.ac.uk/researchguide/saldridge.html
Search for more papers by this authorDavid J. Willock Dr.
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorGlesni A. Pierce
Department of Inorganic Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK, Fax: (+44) 186-527-2690 http://www.chem.ox.ac.uk/researchguide/saldridge.html
Search for more papers by this authorSimon Aldridge Dr.
Department of Inorganic Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK, Fax: (+44) 186-527-2690 http://www.chem.ox.ac.uk/researchguide/saldridge.html
Search for more papers by this authorCameron Jones Prof.
School of Chemistry, Monash University, PO Box 23, Victoria 3800, Australia
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorTimo Gans-Eichler Dr.
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorAndreas Stasch Dr.
School of Chemistry, Monash University, PO Box 23, Victoria 3800, Australia
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorNatalie D. Coombs
Department of Inorganic Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK, Fax: (+44) 186-527-2690 http://www.chem.ox.ac.uk/researchguide/saldridge.html
Search for more papers by this authorDavid J. Willock Dr.
Cardiff School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK
Search for more papers by this authorWe thank the EPSRC for funding and for access to the National Mass Spectrometry facility. C.J. would like to acknowledge funding from the ARC.
Graphical Abstract
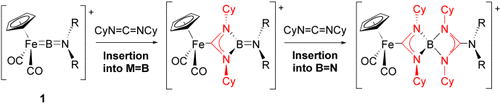
One thing leads to another: Reactions of the cationic BN vinylidene analogues 1-[BArF4] (R=Cy, iPr; ArF=3,5-(CF3)2C6H3) towards dicyclohexylcarbodiimide proceed by unprecedented insertion chemistry for terminal borylene complexes. Controlled, stepwise insertion into the FeB and BN bonds is demonstrated, sequentially forming four-membered rings linked at a spirocyclic boronium center.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z604838_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. P. Collman, L. S. Hegedus, J. R. Norton, R. G. Finke, Principles and Applications of Organotransition Metal Chemistry, University Science Books, Sausalito, 1987.
- 2For a recent review of mechanistic aspects of olefin polymerization, see, for example: M. Bochmann, J. Organomet. Chem. 2004, 689, 3982–3998.
- 3For a recent review of mechanistic aspects of hydroformylation chemistry, see, for example: P. C. J. Kamer, A. van Rooy, G. C. Schoemaker, P. W. N. M. van Leeuwen, Coord. Chem. Rev. 2004, 248, 2409–2424.
- 4For a recent review of Fischer carbene complexes, which includes aspects of alkyne insertion into MC double bonds and the Dötz reaction, see, for example: A. de Meijere, H. Schirmer, M. Duetsch, Angew. Chem. 2000, 112, 4124–4162;
10.1002/1521-3757(20001117)112:22<4124::AID-ANGE4124>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3964–4002, and references therein.10.1002/1521-3773(20001117)39:22<3964::AID-ANIE3964>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 5For a recent review, see, for example: T. B. Marder, N. C. Norman, Top. Catal. 1998, 5, 63–73.
- 6For recent reviews of borylene chemistry, see:
- 6aH. Braunschweig, M. Colling, Eur. J. Inorg. Chem. 2003, 393–403;
- 6bS. Aldridge, D. L. Coombs, Coord. Chem. Rev. 2004, 248, 535–559;
- 6cH. Braunschweig, Adv. Organomet. Chem. 2004, 51, 163–192;
- 6dH. Braunschweig, D. Rais, Heteroat. Chem. 2005, 16, 566–571;
- 6eH. Braunschweig, C. Kollann, D. Rais, Angew. Chem. 2006, 118, 5380–5400; Angew. Chem. Int. Ed. 2006, 45, 5254–5274.
- 7See, for example:
- 7aW. A. Nugent, J. M. Mayer, Metal Ligand Multiple Bonds, Wiley-Interscience, New York, 1988;
- 7bR. R. Schrock, J. Chem. Soc. Dalton Trans. 2001, 2541–2550;
- 7cT. M. Trnka, R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18–29;
- 7dG. P. Mitchell, T. D. Tilley, J. Am. Chem. Soc. 1997, 119, 11236–11243.
- 8
- 8aD. L. Coombs, S. Aldridge, C. Jones, D. J. Willock, J. Am. Chem. Soc. 2003, 125, 6356–6357;
- 8bD. L. Coombs, S. Aldridge, A. Rossin, C. Jones, D. J. Willock, Organometallics 2004, 23, 2911–2926.
- 9
- 9aH. Braunschweig, M. Colling, C. Kollann, H. G. Stammler, B. Neumann, Angew. Chem. 2001, 113, 2359–2361;
Angew. Chem. Int. Ed. 2001, 40, 2298–2300; ;
10.1002/1521-3773(20010618)40:12<2298::AID-ANIE2298>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 9bH. Braunschweig, M. Colling, C. Hu, K. Radacki, Angew. Chem. 2003, 115, 215–218; Angew. Chem. Int. Ed. 2003, 42, 205–208;
- 9cH. Braunschweig, D. Rais, K. Uttinger, Angew. Chem. 2005, 117, 3829–3832; Angew. Chem. Int. Ed. 2005, 44, 3763–3766;
- 9dH. Braunschweig, K. Radacki, D. Rais, F. Seeler, Angew. Chem. 2006, 118, 1087–1090; Angew. Chem. Int. Ed. 2006, 45, 1066–1069;
- 9eH. Braunschweig, M. Forster, K. Radacki, Angew. Chem. 2006, 118, 2187–2189; Angew. Chem. Int. Ed. 2006, 45, 2132–2134.
- 10
- 10aH. Braunschweig, T. Herbst, D. Rais, F. Seeler, Angew. Chem. 2005, 117, 7627–7629; Angew. Chem. Int. Ed. 2005, 44, 7461–7463; ;
- 10bS. Aldridge, C. Jones, T. Gans-Eichler, A. Stasch, D. L. Kays (née Coombs), N. D. Coombs, D. J. Willock, Angew. Chem. 2005, 117, 6264–6268; Angew. Chem. Int. Ed. 2006, 45, 6118–6122.
- 11D. L. Kays (née Coombs), J. K. Day, L.-L. Ooi, S. Aldridge, Angew. Chem. 2005, 117, 7623–7626;
10.1002/ange.200502343 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7457–7460; .
- 12D. L. Kays (née Coombs), J. K. Day, S. Aldridge, R. W. Harrington, W. Clegg, Angew. Chem. 2006, 118, 3593–3596;
10.1002/ange.200600714 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3513–3516.
- 13For a recent review of boron-centered cations, see: W. E. Piers, S. C. Bourke, K. D. Conroy, Angew. Chem. 2005, 117, 5142–5163;
10.1002/ange.200500402 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5016–5036.
- 14For a related boronium system, see: K. B. Anderson, R. A. Franich, H. W. Kroese, R. Meder, C. E. F. Rickard, Polyhedron 1995, 14, 1149–1153.
- 15D. J. Brauer, S. Buchheim-Speigel, H. Bürger, R. Gielen, G. Pawelke, J. Rothe, Organometallics 1997, 16, 5321–5330.
- 16For related boron amidinate systems, see, for example:
- 16aN. J. Hill, M. Findlater, A. H. Cowley, Dalton Trans. 2005, 3229–3234;
- 16bM. Findlater, N. J. Hill, A. H. Cowley, Polyhedron 2006, 25, 983–988.
- 17For comparison, the corresponding torsion angle in Cy2NC(NCy)2BCl2 is 28.3°: S. Aldridge, G. A. Pierce, unpublished results.
- 18H. Brunner, W. Meier, J. Wachter, I. Bernal, E. Raabe, J. Organomet. Chem. 1989, 362, 95–103.
- 19As determined from a survey of the Cambridge Crystallographic Database in November, 2006. By means of comparison, the FeC bond length in [Cp(OC)2FeCHCHCHCHBr] is 1.971(3) Å: R. Ferede, M. Noble, A. W. Cordes, N. T. Allison, J. Lay, Jr., J. Organomet. Chem. 1988, 339, 1–6.
- 20N. J. Hill, J. A. Moore, M. Findlater, A. H. Cowley, Chem. Commun. 2005, 5462–5464.
- 21G. J. Irvine, M. J. G. Lesley, T. B. Marder, N. C. Norman, C. R. Rice, E. G. Robins, W. R. Roper, G. R. Whittell, L. J. Wright, Chem. Rev. 1998, 98, 2685–2722.
- 22See, for example: L. H. Gade, H. Memmler, U. Kauper, A. Schneider, S. Fabre, I. Bezougli, M. Lutz, C. Galka, I. J. Scowen, M. McPartlin, Chem. Eur. J. 2000, 6, 692–708.
10.1002/(SICI)1521-3765(20000218)6:4<692::AID-CHEM692>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 23DFT calculations performed on the [CpFe(CO)2C(NCy)2BNCy2]+ ion, using a precedented methodology, reveal an 85:15 σ/π breakdown of the bonding density for the metalla-amidinate FeC bond, compared to 64:36 for the model carbene system [CpFe(CO)2(CH2)]+:
- 23aS. Aldridge, A. Rossin, D. L. Coombs, D. J. Willock, Dalton Trans. 2004, 2649–2654;
- 23bA. A. Dickinson, D. J. Willock, R. J. Calder, S. Aldridge, Organometallics 2002, 21, 1146–1157.
- 24R. Jefferson, M. F. Lappert, B. Prokai, B. P. Tilley, J. Chem. Soc. A 1966, 1584–1590.