Pd-Catalyzed Cleavage of Benzylic Nitro Bonds: New Opportunities for Asymmetric Synthesis†
Thomas C. Fessard Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorHajime Motoyoshi Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorErick M. Carreira Prof. Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorThomas C. Fessard Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorHajime Motoyoshi Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorErick M. Carreira Prof. Dr.
Laboratorium für Organische Chemie, ETH Hönggerberg, 8093 Zürich, Switzerland, Fax: (+41) 1-632-1328
Search for more papers by this authorThis work was supported by the ETH Zürich (INIT) and the Uehara Memorial Foundation (financial support to H.M.).
Graphical Abstract
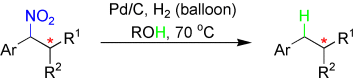
Without a trace: Benzylic nitroalkanes are reduced to the corresponding parent alkanes in good yields by using a simple procedure involving heterolytic CN bond cleavage (see scheme). Traceless removal of the nitro group leaves behind a stereogenic center that may otherwise be difficult to install. This reaction significantly expands the scope of building blocks that can be accessed.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z604263_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Ono, The Nitro Group in Organic Synthesis Wiley-VCH, New York, 2001, p. 392;
10.1002/0471224480 Google Scholar
- 1bD. Seebach, E. W. Colvin, F. Lehr, T. Weller, Chimia 1979, 33, 1.
- 2C. Czekelius, E. M. Carreira, Angew. Chem. 2005, 117, 618;
10.1002/ange.200461879 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 612.
- 3
- 3aA. Kamimura, K. Kurata, N. Ono, Tetrahedron Lett. 1989, 30, 4819;
- 3bN. Ono, T. Hashimoto, T. X. Jun, A. Kaji, Tetrahedron Lett. 1987, 28, 2277;
- 3cH. Suzuki, K. Takaoka, A. Osuka, Bull. Chem. Soc. Jpn. 1985, 58, 1067;
- 3dG. Rosini, R. Ballini, Synthesis 1983, 137;
- 3eN. Ono, H. Miyake, R. Tamura, A. Kaji, Tetrahedron Lett. 1981, 22, 1705;
- 3fN. Ono, R. Tamura, A. Kaji, J. Am. Chem. Soc. 1980, 102, 2851;
- 3gN. Kornblum, S. C. Carlson, R. G. Smith, J. Am. Chem. Soc. 1979, 101, 647;
- 3hA. L. Krasuska, H. Pitrowska, T. Urbanski, Tetrahedron Lett. 1979, 20, 1243;
10.1016/S0040-4039(01)86114-1 Google Scholar
- 3iN. Kornblum, Angew. Chem. 1975, 87, 797; Angew. Chem. Int. Ed. Engl. 1975, 14, 734.
- 4J. Tormo, D. H. Hays, G. C. Fu, J. Org. Chem. 1998, 63, 5296. We have subjected 1 to these catalytic conditions, although less than 50 % conversion into 2 was observed after 24 h.
- 5
- 5aC. Jousse-Karinthi, C. Riche, A. Chiaroni, D. Desmaele, Eur. J. Org. Chem. 2001, 3631;
- 5bP. M. G. Bavin, J. Med. Chem. 1966, 9, 52;
- 5cM. Hamana, M. Yamazaki, Chem. Pharm. Bull. 1963, 11, 415.
- 6
- 6aN. Ono, I. Hamamoto, A. Kamimura, A. Kaji, J. Org. Chem. 1986, 51, 3734;
- 6bfor the single example of a reaction on a tertiary benzylic substrate, see: F. J. Stiefel, U.S. 486837, 1989.
- 7For reviews, see:
- 7aC. D. Weis, G. R. Newkome, Synthesis 1995, 1053;
- 7bN. Ono, A. Kaji, Synthesis 1986, 693.
- 8
- 8aJ. C. Borah, S. Gogoi, J. Boruwa, B. Kalita, N. C. Barua, Tetrahedron Lett. 2004, 45, 3689;
- 8bA. Kamimura, N. Ono, Tetrahedron Lett. 1989, 30, 731.
- 9M. Yamashita, K.-I. Yamada, K. Tomioka, Org. Lett. 2005, 7, 2369.
- 10J. S. Costa, A. G. Dias, A. L. Anholeto, M. D. Monteiro, V. L. Patrocinio, P. R. R. Costa, J. Org. Chem. 1997, 62, 4002.
- 11
- 11aH. Sasai, T. Suzuki, N. Itoh, M. Shibasaki, Tetrahedron Lett. 1993, 34, 851;
- 11bH. Sasai, N. Itoh, T. Suzuki, M. Shibasaki, Tetrahedron Lett. 1993, 34, 855.
- 12The fact that deuterium incorporation does not occur at the benzylic site renders the process quite different to that observed with tertiary allylic organonitro compounds, in which the organonitro substrate leads to an organopalladium intermediate which can be intercepted by a range of nucleophiles including hydrides; see reference [6a].
- 13Y. Yamamoto, I. Nakamura, Top. Organomet. Chem. 2005, 14, 211.
- 14For examples of asymmetric reactions which involve nitroalkanes, see:
- 14aM. Terada, H. Ube, Y. Yaguchi, J. Am. Chem. Soc. 2006, 128, 1454, and references [8] and [9] therein;
- 14bH. Li, B. Wang, L. Deng, J. Am. Chem. Soc. 2006, 128, 732;
- 14cC. Palomo, M. Oiarbide, R. Halder, A. Laso, R. López, Angew. Chem. 2006, 118, 123;
10.1002/ange.200502674 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 117;
- 14dJ. C. Anderson, G. P. Howell, R. M. Lawrence, C. S. Wilson, J. Org. Chem. 2005, 70, 5665;
- 14eI. Kudyba, J. Raczko, J. Jurczak, J. Org. Chem. 2004, 69, 2844;
- 14fC. Christensen, K. Juhl, R. G. Hazell, K. A. Jørgensen, J. Org. Chem. 2002, 67, 4875;
- 14gK. R. Knudsen, T. Risgaard, N. Nishiwaki, K. V. Gothelf, K. A. Jørgensen, J. Am. Chem. Soc. 2001, 123, 5843;
- 14hN. Nishiwaki, K. R. Knudsen, K. V. Gothelf, K. A. Jørgensen, Angew. Chem. 2001, 113, 3080;
10.1002/1521-3757(20010817)113:16<3080::AID-ANGE3080>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2992;10.1002/1521-3773(20010817)40:16<2992::AID-ANIE2992>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 14iB. M. Trost, J.-P. Surivet, J. Am. Chem. Soc. 2000, 122, 6291;
- 14jK.-i. Yamada, S. J. Harwood, H. Gröger, M. Shibasaki, Angew. Chem. 1999, 111, 3713;
10.1002/(SICI)1521-3757(19991203)111:23<3713::AID-ANGE3713>3.0.CO;2-Z Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3504.10.1002/(SICI)1521-3773(19991203)38:23<3504::AID-ANIE3504>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 15Y. Sohtome, Y. Hashimoto, K. Nagasawa, Eur. J. Org. Chem. 2006, 2894.
- 16For examples of asymmetric reactions which involve nitro-olefins, see:
- 16aD. Enders, M. R. M. Hüttl, C. Grondal, G. Raabe, Nature 2006, 441, 861;
- 16bH. Huang, E. N. Jacobsen, J. Am. Chem. Soc. 2006, 128, 7170;
- 16cN. Mase, K. Watanabe, H. Yoda, K. Takabe, C. F. Barbas III, J. Am. Chem. Soc. 2006, 128, 4966;
- 16dA. E. Mattson, A. M. Zuhl, T. E. Reynolds, K. A. Scheidt, J. Am. Chem. Soc. 2006, 128, 4932;
- 16eC. Palomo, S. Vera, A. Mielgo, E. Gomez-Bengoa, Angew. Chem. 2006, 118, 6130;
10.1002/ange.200602207 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5984;
- 16fJ. Wang, H. Li, B. Lou, L. Zu, H. Guo, W. Wang, Chem. Eur. J. 2006, 12, 4321;
- 16gS. Mossé, A. Alexakis, Org. Lett. 2006, 8, 3577;
- 16hL. Zu, J. Wang, H. Li, W. Wang, Org. Lett. 2006, 8, 3077;
- 16iS.-F. Lu, D.-M. Du, J. Xu, Org. Lett. 2006, 8, 2115;
- 16jD. A. Evans, S. Seidel, J. Am. Chem. Soc. 2005, 127, 9958;
- 16kJ. Wu, D. M. Mampreian, A. H. Hoveyda, J. Am. Chem. Soc. 2005, 127, 4584;
- 16lR. P. Herrera, V. Sgarzani, L. Bernardi, A. Ricci, Angew. Chem. 2005, 117, 6734;
10.1002/ange.200500227 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6576;
- 16mA. J. A. Cobb, D. M. Shaw, D. A. Longbottom, J. B. Gold, S. V. Ley, Org. Biomol. Chem. 2005, 3, 84;
- 16nF. Valleix, K. Nagai, T. Soeta, M. Kuriyama, K.-I. Yamada, K. Tomioka, Tetrahedron 2005, 61, 7420;
- 16oD. Polet, A. Alexakis, Tetrahedron Lett. 2005, 46, 1529;
- 16pW. Notz, F. Tanaka, C. F. Barbas III, Acc. Chem. Res. 2004, 37, 580;
- 16qA. Alexakis, D. Polet, S. Rosset, S. March, J. Org. Chem. 2004, 69, 5660;
- 16rD. M. Mampreian, A. H. Hoveyda, Org. Lett. 2004, 6, 2829;
- 16sH. Choi, Z. Hua, I. Ojima, Org. Lett. 2004, 6, 2689;
- 16tN. J. Adderley, D. J. Buchanan, D. J. Dixon, D. I. Laine, Angew. Chem. 2003, 115, 4373;
10.1002/ange.200352007 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4241;
- 16uJ. Kang, J. H. Lee, D. S. Lim, Tetrahedron: Asymmetry 2003, 14, 305;
- 16vM. Molteni, A. Volonterio, M. Zanda, Org. Lett. 2003, 5, 3887;
- 16wA. Alexakis, C. Benhaim, S. Rosset, M. Humam, J. Am. Chem. Soc. 2002, 124, 5262;
- 16xA. Duursma, A. J. Minnaard, B. L. Feringa, Tetrahedron 2002, 58, 5773;
- 16yC. A. Luchaco-Cullis, A. H. Hoveyda, J. Am. Chem. Soc. 2002, 124, 8192;
- 16zJ. M. Betancort, C. F. Barbas III, Org. Lett. 2001, 3, 3737;
- 16aaS. Ongeri, U. Piarulli, R. F. W. Jackson, C. Gennari, Eur. J. Org. Chem. 2001, 803;
- 16abA. Alexakis, C. Benhaim, Org. Lett. 2000, 2, 2579;
- 16acD. Enders, A. Haertwig, G. Raabe, J. Runsink, Eur. J. Org. Chem. 1998, 1771;
10.1002/(SICI)1099-0690(199809)1998:9<1771::AID-EJOC1771>3.0.CO;2-P CAS Web of Science® Google Scholar
- 16adN. Sewald, V. Wendisch, Tetrahedron: Asymmetry 1998, 9, 1341;
- 16aeD. Lucet, L. Toupet, T. Le Gall, C. Mioskowski, J. Org. Chem. 1997, 62, 2682;
- 16afD. Enders, A. Härtwig, G. Raabe, J. Runsink, Angew. Chem. 1996, 108, 2540;
10.1002/ange.19961082026 Google ScholarAngew. Chem. Int. Ed. 1996, 35, 2388.
- 17Compounds of the class represented by 1 can also be accessed enantioselectively by conjugate addition of a phenyl lithium species to (E)-(1-nitrobut-1-enyl)benzene in the presence of sparteine; see:
- 17aM. Yamahita, K. Yamada, K. Tomioka, J. Am. Chem. Soc. 2004, 126, 1954;
- 17bM. Yamashita, K. Yamada, K. Tomioka, Tetrahedron 2004, 60, 4237.
- 18In a related approach which involves β-amido nitroalkanes, the nitro group was reduced using stoichiometric amounts of Bu3SnH and AIBN; see: M.-L. Leroux, T. Le Gall, C. Mioskowski, Tetrahedron: Asymmetry 2001, 12, 1817; see also M. Petrini, E. Torregiani, Tetrahedron Lett. 2006, 47, 3501.
- 19
- 19aC. Czekelius, E. M. Carreira, Org. Lett. 2004, 6, 4575;
- 19bC. Czekelius, E. M. Carreira, Angew. Chem. 2003, 115, 4941;
10.1002/ange.200352175 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4793.
- 20
- 20aE. M. Vogl, S. L. Buchwald, J. Org. Chem. 2002, 67, 106–111;
- 20bJ. M. Fox, X. Huang, A. Chieffi, S. L. Buchwald, J. Am. Chem. Soc. 2000, 122, 1360.