Aryl Calcium Compounds: Syntheses, Structures, Physical Properties, and Chemical Behavior
Matthias Westerhausen
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorMartin Gärtner
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorReinald Fischer
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorJens Langer
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorMatthias Westerhausen
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorMartin Gärtner
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorReinald Fischer
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorJens Langer
Institute of Inorganic and Analytical Chemistry, Friedrich-Schiller-Universität Jena, August-Bebel-Strasse 2, 07743 Jena, Germany, Fax: (+49) 3641-948-102
Search for more papers by this authorGraphical Abstract
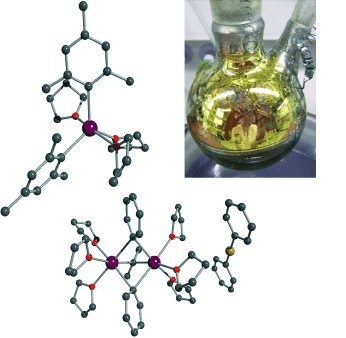
Calcium supplements for Grignard: The easy access of aryl calcium compounds in high yields now offers the possibility of investigating the properties and chemical behavior of these heavy Grignard reagents (see picture; C black, Ca purple, Cu yellow, O red). The key points are the nature of activation of the metal prior to use and the use of low reaction and handling temperatures to prevent side and decomposition reactions.
Abstract
Organocalcium chemistry is still in its infancy. The direct synthesis of activated calcium and (substituted) iodobenzenes allows for the large-scale and high-yield synthesis of aryl calcium iodides. The influence of the substitution patterns of the phenyl group, halogen atom, and solvent is discussed. Aryl calcium iodides show a Schlenk equilibrium that enables the isolation of diaryl calcium derivatives. Owing to the high reactivity of aryl calcium halides, low temperatures have to be maintained throughout the preparative procedures in order to avoid side reactions. A decrease of reactivity and, hence, an enhanced stability at higher temperatures can be achieved by shielding of the calcium atom by increasing the coordination number of the metal center or by substitution of the iodide anion by bulky groups.
References
- 1D. Seyferth, Organometallics 2006, 25, 2–24.
- 2
- 2aW. N. Setzer, P. von R. Schleyer, Adv. Organomet. Chem. 1985, 24, 353–451;
- 2bC. Schade, P. von R. Schleyer, Adv. Organomet. Chem. 1987, 27, 169–278.
- 3
- 3aC. Elschenbroich, A. Salzer, Organometallics: A Concise Introduction, 2nd ed., Weinheim, VCH, 1992;
- 3b Grignard Reagents New Developments (Ed.: ), Wiley, Chichester, 2000.
- 4B. G. Gowenlock, W. E. Lindsell, J. Organomet. Chem. Libr. 1977, 1–73.
- 5J. J. Eisch, R. B. King, Organometallic Synthesis, Vol. 2, Academic Press, New York, 1981, p. 101.
10.1016/B978-0-12-234950-8.50014-X Google Scholar
- 6R. Zerger, G. Stucky, J. Organomet. Chem. 1974, 80, 7–17.
- 7
- 7aP. Jutzi, Adv. Organomet. Chem. 1986, 26, 217–295;
- 7bP. Jutzi, J. Organomet. Chem. 1990, 400, 1–17;
- 7cT. P. Hanusa, Polyhedron 1990, 9, 1345–1362;
- 7dT. P. Hanusa, Chem. Rev. 1993, 93, 1023–1036;
- 7eD. J. Burkey, T. P. Hanusa, Comments Inorg. Chem. 1995, 17, 41–77;
- 7fP. Jutzi, N. Burford in Metallocenes (Eds.: ), Wiley-VCH, Weinheim, 1998, chap. 1, pp. 3–54;
- 7gM. L. Hays, T. P. Hanusa, Adv. Organomet. Chem. 1996, 40, 117–170;
- 7hP. Jutzi, N. Burford, Chem. Rev. 1999, 99, 969–990;
- 7iT. P. Hanusa, Organometallics 2002, 21, 2559–2571.
- 8
- 8aT. P. Hanusa, Coord. Chem. Rev. 2000, 210, 329–367;
- 8bM. Westerhausen, Angew. Chem. 2001, 113, 3063–3065;
10.1002/1521-3757(20010817)113:16<3063::AID-ANGE3063>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2975–2977;10.1002/1521-3773(20010817)40:16<2975::AID-ANIE2975>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 8cJ. S. Alexander, K. Ruhlandt-Senge, Eur. J. Inorg. Chem. 2002, 2761–2774.
- 9F. G. N. Cloke, P. B. Hitchcock, M. F. Lappert, G. A. Lawless, B. Royo, J. Chem. Soc. Chem. Commun. 1991, 724–726.
- 10C. Eaborn, S. A. Hawkes, P. B. Hitchcock, J. D. Smith, Chem. Commun. 1997, 1961–1962.
- 11
- 11aF. Feil, S. Harder, Organometallics 2000, 19, 5010–5015;
- 11bS. Harder, F. Feil, A. Weeber, Organometallics 2001, 20, 1044–1046;
- 11cS. Harder, F. Feil, Organometallics 2002, 21, 2268–2274;
- 11dF. Feil, C. Müller, S. Harder, J. Organomet. Chem. 2003, 683, 56–63;
- 11eS. Harder, S. Müller, E. Hübner, Organometallics 2004, 23, 178–183.
- 12V. Knapp, G. Müller, Angew. Chem. 2001, 113, 187–190;
10.1002/1521-3757(20010105)113:1<187::AID-ANGE187>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2001, 40, 183–186.10.1002/1521-3773(20010105)40:1<183::AID-ANIE183>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 13E. Beckmann, Ber. Dtsch. Chem. Ges. 1905, 38, 904–906.
- 14P. R. Markies, T. Nomoto, G. Schat, O. S. Akkerman, F. Bickelhaupt, W. J. J. Smeets, A. L. Spek, Organometallics 1991, 10, 3826–3837.
- 15
- 15aK. Mochida, H. Ogawa, J. Organomet. Chem. 1983, 243, 131–135;
- 15bK. Mochida, Y. Hiraga, H. Takeuchi, H. Ogawa, Organometallics 1987, 6, 2293–2297;
- 15cJ. P. Dunne, M. Tacke, C. Selinka, D. Stalke, Eur. J. Inorg. Chem. 2003, 1416–1425.
- 16S. Harder, J. Brettar, Angew. Chem. 2006, 118, 3554–3558; Angew. Chem. Int. Ed. 2006, 45, 3474–3478.
- 17C. Ruspic, S. Harder, Organometallics 2005, 24, 5506–5508.
- 18R. Fischer, H. Görls, M. Westerhausen, Inorg. Chem. Commun. 2005, 8, 1159–1161.
- 19S.-O. Hauber, F. Lissner, G. B. Deacon, M. Niemeyer, Angew. Chem. 2005, 117, 6021–6025;
10.1002/ange.200501494 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5871–5875.
- 20M. Westerhausen, Coord. Chem. Rev. 1998, 176, 157–210.
- 21
- 21aW. C. Johnson, M. F. Stubbs, A. E. Sidwell, A. Pechukas, J. Am. Chem. Soc. 1939, 61, 318–329;
- 21bW. J. McCreary, J. Met. 1958, 10, 615–617;
- 21cJ. Evers, A. Weiss, E. Kaldis, J. Muheim, J. Less-Common Met. 1973, 30, 83–95.
- 22S. R. Drake, D. J. Otway, J. Chem. Soc. Chem. Commun. 1991, 517–519; Erratum: S. R. Drake, D. J. Otway, J. Chem. Soc. Chem. Commun. 1991, 1060.
- 23H. Bönnemann, B. Bogdanovic, R. Brinkmann, N. Egeler, R. Benn, I. Topalovic, K. Seevogel, Main Group Met. Chem. 1990, 13, 341–362.
- 24
- 24aR. D. Rieke, Science 1989, 246, 1260–1264;
- 24bT.-C. Wu, H. Xiong, R. D. Rieke, J. Org. Chem. 1990, 55, 5045–5051;
- 24cM. J. McCormick, K. B. Moon, S. R. Jones, T. P. Hanusa, J. Chem. Soc. Chem. Commun. 1990, 778–779.
- 25K. J. Klabunde, Acc. Chem. Res. 1975, 8, 393–399.
- 26D. C. Bradley, M. B. Hursthouse, A. A. Ibrahim, K. M. Abdul Malik, M. Motevalli, R. Möseler, H. Powell, J. D. Runnacles, A. C. Sullivan, Polyhedron 1990, 9, 2959–2964.
- 27
- 27aJ. S. Alexander, K. Ruhlandt-Senge, Angew. Chem. 2001, 113, 2732–2734;
10.1002/1521-3757(20010716)113:14<2732::AID-ANGE2732>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2658–2660;10.1002/1521-3773(20010716)40:14<2658::AID-ANIE2658>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 27bJ. S. Alexander, K. Ruhlandt-Senge, H. Hope, Organometallics 2003, 22, 4933–4937.
- 28R. Fischer, M. Gärtner, H. Görls, M. Westerhausen, Organometallics 2006, 25, 3496–3500.
- 29R. Fischer, M. Gärtner, H. Görls, M. Westerhausen, Angew. Chem. 2006, 118, 624–627; Angew. Chem. Int. Ed. 2006, 45, 609–612.
- 30M. Gärtner, H. Görls, M. Westerhausen, Synthesis 2007, in press.
- 31R. Fischer, M. Gärtner, H. Görls, L. Yu, M. Reiher, M. Westerhausen, Angew. Chem., ; Angew. Chem. Int. Ed., .
- 32J. Langer, M. Westerhausen, unpublished results.
- 33M. Gärtner, H. Görls, M. Westerhausen, Organometallics 2007, in press.
- 34
- 34aP. B. Hitchcock, M. F. Lappert, G. A. Lawless, B. Royo, J. Chem. Soc. Chem. Commun. 1990, 1141–1143;
- 34bK. F. Tesh, T. P. Hanusa, J. C. Huffman, C. J. Huffman, Inorg. Chem. 1992, 31, 5572–5579.
- 35M. Westerhausen, M. Hartmann, N. Makropoulos, B. Wieneke, M. Wieneke, W. Schwarz, D. Stalke, Z. Naturforsch. B 1998, 53, 117–125.
- 36M. Westerhausen, W. Schwarz, Z. Anorg. Allg. Chem. 1991, 604, 127–140.
- 37M. Westerhausen, W. Schwarz, Z. Naturforsch. B 1992, 47, 453–459.
- 38J. J. Ritter, R. D. Anderson, J. Org. Chem. 1959, 24, 208–210; see also: F. W. Swamer, G. A. Reynolds, C. R. Hauser, J. Org. Chem. 1951, 16, 43–46.
- 39M. Westerhausen, Dalton Trans. 2006, 4755–4768.
- 40M. Westerhausen, C. Gückel, H. Piotrowski, M. Vogt, Z. Anorg. Allg. Chem. 2002, 628, 735–740.
- 41R. Fischer, M. Westerhausen, unpublished results.
- 42
- 42aW. Biltz, G. F. Hüttig, Z. Anorg. Allg. Chem. 1920, 114, 241–265;
- 42bR. Juza, H. Schumacher, Z. Anorg. Allg. Chem. 1963, 324, 278–286;
- 42cR. Juza, Angew. Chem. 1964, 76, 290–300;
- 42dN. Mammano, M. J. Sienko, J. Solid State Chem. 1970, 1, 534–535.
10.1016/0022-4596(70)90138-6 Google Scholar
- 43G. Bruhat, V. Thomas, C. R. Hebd. Seances Acad. Sci. 1926, 183, 297–299.
- 44M. Fossatelli, R. den Besten, H. D. Verkruijsse, L. Brandsma, Recl. Trav. Chim. Pays-Bas 1994, 113, 527–528.
- 45K. B. Dillon, F. Mathey, J. F. Nixon, Phosphorus: The Carbon Copy, Chichester, Wiley, 1998.
- 46M. Westerhausen, M. H. Digeser, H. Nöth, T. Seifert, A. Pfitzner, J. Am. Chem. Soc. 1998, 120, 6722–6725.
- 47M. Westerhausen, M. H. Digeser, H. Nöth, W. Ponikwar, T. Seifert, K. Polborn, Inorg. Chem. 1999, 38, 3207–3214.