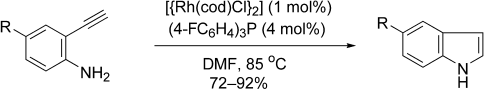Rhodium-Catalyzed Cycloisomerization: Formation of Indoles, Benzofurans, and Enol Lactones†
We thank the National Science Foundation and the National Institutes of Health, General Medical Sciences Institute (GM 33049) for their generous support of our programs. Mass spectra were provided by the Mass Spectrometry Regional Center of the University of California, San Francisco, which is supported by the NIH Division of Research Resources.
Graphical Abstract
Internal affairs: Indoles, benzofurans, and enol lactones are formed chemoselectively from the rhodium-catalyzed cycloisomerization reaction of easily prepared alkynyl aniline substrates (see scheme, cod=cycloocta-1,5-diene, DMF=N,N-dimethylformamide). The reaction may proceed by nucleophilic capture of a vinylidene intermediate. Indoles are formed under mild conditions using low catalyst loadings.