Copper-Catalyzed Cross-Coupling Reaction of Grignard Reagents with Primary-Alkyl Halides: Remarkable Effect of 1-Phenylpropyne†
Jun Terao Dr.
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorHirohisa Todo
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorShameem Ara Begum
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorHitoshi Kuniyasu Dr.
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorNobuaki Kambe Prof. Dr.
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorJun Terao Dr.
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorHirohisa Todo
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorShameem Ara Begum
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorHitoshi Kuniyasu Dr.
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorNobuaki Kambe Prof. Dr.
Department of Applied Chemistry & Science and Technology Center for Atoms, Molecules, and Ions Control, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan, Fax: (+81) 6-6879-7390
Search for more papers by this authorThis study was supported by the Industrial Technology Research Grant Program in 2006 from the New Energy and Industrial Technolgy Development Organization (NEDO) of Japan and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Graphical Abstract
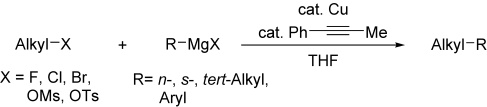
A general get-together: The Cu-catalyzed cross-coupling reaction of primary-alkyl halides with primary-, secondary-, and tertiary-alkyl and phenyl Grignard reagents proceeds efficiently in THF under reflux in the presence of 1-phenylpropyne (see scheme). The reaction is also applicable to alkyl mesylates (OMs) and tosylates (OTs). The reactivities of alkylX with a Grignard reagent increase in the order X=Cl<F<OMs<OTs<Br.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2007/z603451_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. H. Lipshutz, S. Sengupta, Organic Reactions, Vol. 41 (Ed.: ), Wiley, New York, 1992, p. 149.
- 2For recent reviews on transition-metal-catalyzed cross-coupling reactions using alkyl halides, see:
- 2aD. J. Cárdenas, Angew. Chem. 1999, 111, 3201;
10.1002/(SICI)1521-3757(19991018)111:20<3201::AID-ANGE3201>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3018;10.1002/(SICI)1521-3773(19991018)38:20<3018::AID-ANIE3018>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 2bT.-Y. Luh, M.-K. Leung, K.-T. Wong, Chem. Rev. 2000, 100, 3187;
- 2cD. J. Cárdenas, Angew. Chem. 2003, 115, 557;
10.1002/ange.200390091 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 384;
- 2dM. R. Netherton, G. C. Fu, Adv. Synth. Catal. 2004, 346, 1525;
- 2eJ. Terao, N. Kambe, J. Synth. Org. Chem. Jpn. 2004, 62, 1192;
- 2fA. C. Frisch, M. Beller, Angew. Chem. 2005, 117, 680; Angew. Chem. Int. Ed. 2005, 44, 674.
- 3
- 3aG. Cahiez, S. Marquais, Synlett 1993, 45;
- 3bG. Cahiez, C. Chaboche, M. Jezequel, Tetrahedron 2000, 56, 2733.
- 4For transition-metal-catalyzed cross-coupling reactions using alkyl chlorides, see
- 4aJ. Terao, H. Watanabe, A. Ikumi, H. Kuniyasu, N. Kambe, J. Am. Chem. Soc. 2002, 124, 4222;
- 4bJ. H. Kirchhoff, C. Dai, G. C. Fu, Angew. Chem. 2002, 114, 2025;
Angew. Chem. Int. Ed. 2002, 41, 1945;
10.1002/1521-3773(20020603)41:11<1945::AID-ANIE1945>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 4cA. C. Frisch, N. Shaikh, A. Zapf, M. Beller, Angew. Chem. 2002, 114, 4218;
Angew. Chem. Int. Ed. 2002, 41, 4056;
10.1002/1521-3773(20021104)41:21<4056::AID-ANIE4056>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 4dJ. Zhou, G. C. Fu, J. Am. Chem. Soc. 2003, 125, 12527;
- 4eM. Nakamura, K. Matsuo, S. Ito, E. Nakamura, J. Am. Chem. Soc. 2004, 126, 3686.
- 5J. Terao, A. Ikumi, H. Kuniyasu, N. Kambe, J. Am. Chem. Soc. 2003, 125, 5646.
- 6It is known that stoichiometric cuprates such as Li2CuMe3 react with alkyl chlorides and fluorides, see E. C. Ashby, J. J. Lin, J. Org. Chem. 1977, 42, 2805.
- 7In this reaction, 0.09 mmol of 1-phenylpropyne was recovered and a trace amount of PhCHC(Me)nBu (<0.01 mmol) was formed, probably from a Cu-catalyzed carbomagnezation of 1-phenylpropyne with nBuMgCl.
- 8Two examples of Cu-catalyzed cross-coupling reactions using sec-alkyl mesylates have been reported, see D. H. Burns, J. D. Miller, H. K. Chan, M. O. Delaney, J. Am. Chem. Soc. 1997, 119, 2125.
- 9The calculated bond energies of XMgCl obtained by using the G2 method of the Gaussian 98 program are 142, 112, 101 kcal mol−1, and those of XCH3 are 112, 85, 74 kcal mol−1 for X=F, Cl, and Br, respectively. The energy differences between the XMgCl and XCH3 bonds for X=F, Cl, and Br are similar (30, 28, 27 kcal mol−1, respectively), thus indicating that the reaction of alkyl fluorides is not disfavored energetically.
- 10A theoretical study led to a proposed cyclic transition state with an RXLi interaction, which facilitates cleavage of the RX bond in the rate-determining step: E. Nakamura, S. Mori, K. Morokuma, J. Am. Chem. Soc. 2000, 122, 7294.
- 11
- 11aE. C. Ashby, R. N. Depriest, A. Tuncay, S. Srivastava, Tetrahedron Lett. 1982, 23, 5251;
- 11bE. C. Ashby, D. Coleman, J. Org. Chem. 1987, 52, 4554.
- 12The rate constant k=7.0×105 s−1 (at 25 °C) for the isomerization of the 5-hexenyl radical to the cyclopentylmethyl radical has been reported, see A. Citterio, F. Minisci, J. Am. Chem. Soc. 1974, 96, 6355.
- 13The rate constant k=1.3×108 s−1 (at 25 °C) for the isomerization of the cyclopropylmethyl radical to the butenyl radical has been reported, see B. Maillard, D. Forrest, U. K. Ingold, J. Am. Chem. Soc. 1976, 98, 7024.
- 14
- 14aG. M. Whitesides, W. F. Fischer, J. San Filippo, R. W. Bashe, H. O. House, J. Am. Chem. Soc. 1969, 91, 4871;
- 14bC. R. Johnson, G. A. Dutra, J. Am. Chem. Soc. 1973, 95, 7783;
- 14cB. H. Lipshutz, R. S. Wilhelm, J. Am. Chem. Soc. 1982, 104, 4696;
- 14dE. Hebert, Tetrahedron Lett. 1982, 23, 415;
- 14eC.-y. Guo, M. L. Brownawell, J. San Filippo, J. Am. Chem. Soc. 1985, 107, 6028;
- 14fS. Mori, E. Nakamura, K. Morokuma, J. Am. Chem. Soc. 2000, 122, 7294.
- 15It is known that alkylcopper(I) species are formed from both CuCl and CuCl2 on reaction with alkyl Grignard reagents, see M. Tamura, J. K. Kochi, J. Organomet. Chem. 1972, 42, 205.
- 16For thermal stabilities of alkylcopper(I) complexes, see A. Miyashita, T. Yamamoto, A. Yamamoto, Bull. Chem. Soc. Jpn. 1977, 50, 1109, and references therein.
- 17For alkynecopper(I) complexes, see P. Schulte, U. Behrens, J. Organomet. Chem. 1998, 563, 235, and references therein.
- 18
- 18aJ. K. Kochi, Organometallic Mechanisms and Catalysis, Academic Press, New York, 1978, p. 372;
10.1016/B978-0-12-418250-9.50019-X Google Scholar
- 18bJ. M. Klunder, G. H. Posner, Comprehensive Organic Synthesis, Vol. 3 (Eds.: ), Pergamon, Oxford, 1991, p. 207;
10.1016/B978-0-08-052349-1.00062-7 Google Scholar
- 18cE. Nakamura, S. Mori, K. Morokuma, J. Am. Chem. Soc. 1998, 120, 8273.
- 19For bis(alkyne)copper(I) complexes, see G. Gröger, U. Behrens, F. Olbrich, Organometallics 2000, 19, 3354.