Three-Dimensional Chiral Molecule-Based Ferrimagnet with Triple-Helical-Strand Structure†
Hiroyuki Imai Dr.
Department of Applied Molecular Science I, Institute for Molecular Science, Okazaki National Institutes, Myoudaiji, Okazaki, 444-8585, Japan
Present address: Division of Chemistry, Graduate School of Science, Hokkaido University, Sapporo 060-0810, Japan
Search for more papers by this authorKatsuya Inoue Prof. Dr.
Department of Applied Molecular Science I, Institute for Molecular Science, Okazaki National Institutes, Myoudaiji, Okazaki, 444-8585, Japan
Present address: Division of Chemistry, Graduate School of Science, Hiroshima University, Higashi-Hiroshima 739-8526, Japan, Fax: +(81) 82-424-7416
Search for more papers by this authorKohichi Kikuchi Prof. Dr.
Department of Chemistry, Tokyo Metropolitan University, Hachioji, Tokyo 192-0367, Japan
Search for more papers by this authorYusuke Yoshida
Department of Chemistry, Tokyo Metropolitan University, Hachioji, Tokyo 192-0367, Japan
Search for more papers by this authorMitsuhiro Ito
Department of Environmental Technology, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya, Aichi, 466-8555, Japan
Search for more papers by this authorTetsuya Sunahara
Department of Environmental Technology, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya, Aichi, 466-8555, Japan
Search for more papers by this authorSatoru Onaka Prof. Dr.
Department of Environmental Technology, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya, Aichi, 466-8555, Japan
Search for more papers by this authorHiroyuki Imai Dr.
Department of Applied Molecular Science I, Institute for Molecular Science, Okazaki National Institutes, Myoudaiji, Okazaki, 444-8585, Japan
Present address: Division of Chemistry, Graduate School of Science, Hokkaido University, Sapporo 060-0810, Japan
Search for more papers by this authorKatsuya Inoue Prof. Dr.
Department of Applied Molecular Science I, Institute for Molecular Science, Okazaki National Institutes, Myoudaiji, Okazaki, 444-8585, Japan
Present address: Division of Chemistry, Graduate School of Science, Hiroshima University, Higashi-Hiroshima 739-8526, Japan, Fax: +(81) 82-424-7416
Search for more papers by this authorKohichi Kikuchi Prof. Dr.
Department of Chemistry, Tokyo Metropolitan University, Hachioji, Tokyo 192-0367, Japan
Search for more papers by this authorYusuke Yoshida
Department of Chemistry, Tokyo Metropolitan University, Hachioji, Tokyo 192-0367, Japan
Search for more papers by this authorMitsuhiro Ito
Department of Environmental Technology, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya, Aichi, 466-8555, Japan
Search for more papers by this authorTetsuya Sunahara
Department of Environmental Technology, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya, Aichi, 466-8555, Japan
Search for more papers by this authorSatoru Onaka Prof. Dr.
Department of Environmental Technology, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya, Aichi, 466-8555, Japan
Search for more papers by this authorThis work is supported by a Grant-in-Aid for Scientific Research (B) (No. 15340124) from the Ministry of Education, Science, Sports, and Culture. We also thank to Professor Inabe at Hokkaido University for his advice about the crystal structure.
Graphical Abstract
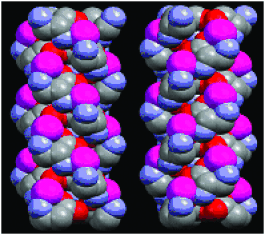
Magnetism gets a new twist: The crystal structure and magnetic properties of a chiral 3D ferrimagnet with Tc of 35 K, [{CrIII(CN)6}{MnII(d- or L-NH2ala)}3]⋅3 H2O (NH2ala=aminoalanine ion) are described. The complex has an extended, chiral, triple-strand helical arrangement of MnII ions, formed by the coordination of the organic ligands (see picture; purple Mn, gray C, blue N, red O). The triple helixes are linked to each other by the {CrIII(CN)6} units.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z460867_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aG. Wagniere, A. Mejer, Chem. Phys. Lett. 1984, 110, 546–551;
- 1bG. L. J. A. Rikken, E. Raupach, Nature 1997, 390, 493–494;
- 1cP. Kleindienst, G. Wagniere, Chem. Phys. Lett. 1998, 288, 89–97;
- 1dG. L. J. A. Rikken, E. Raupach, Phys. Rev. E 1998, 58, 5081–5084.
- 2
- 2aH. Kumagai, K. Inoue, Angew. Chem. 1999, 111, 1694–1696;
10.1002/(SICI)1521-3757(19990601)111:11<1694::AID-ANGE1694>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1601–1603;10.1002/(SICI)1521-3773(19990601)38:11<1601::AID-ANIE1601>3.0.CO;2-# CAS Web of Science® Google Scholar
- 2bH. Kumagai, A. S. Markosyan, K. Inoue, Mol. Cryst. Liq. Cryst. 2000, 40, 97–102;
10.1080/10587250008023509 Google Scholar
- 2cE. Coronado, J. R. Galan-Mascaros, C. J. Gómez-García, J. M. Martinez-Agugo, Inorg. Chem. 2001, 40, 113–120;
- 2dR. Andrés, M. Bissard, M. Gruselle, C. Train, J. Vaissermann, B. Malézieux, J.-P. Jamet, M. Verdaguer, Inorg. Chem. 2001, 40, 4633–4640;
- 2eB. Malézieux, R. Andrés, M. Bissard, M. Gruselle, C. Train, P. Herson, L. L. Troitskaya, V. I. Sokolov, S. T. Ovsenko, T. V. Demeschik, N. S. Ovanesyan, I. A. Mamed'yarova, J. Organomet. Chem. 2001, 637, 182–190;
- 2fD. Armentano, G. D. Munno, F. Lloret, A. V. Palii, M. Julve, Inorg. Chem. 2002, 41, 2007–2013;
- 2gM. Minguet, D. Luneau, E. Lhotel, V. Villar, C. Palusen, D. B. Amabilino, J. Veciana, Angew. Chem. 2002, 114, 606–609;
10.1002/1521-3757(20020215)114:4<606::AID-ANGE606>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 2002, 41, 586–589.
- 3
- 3aJ.-M. Lehn, A. Rigault, J. Siegel, J. Harrowfield, B. Chevrier, D. Moras, Proc. Natl. Acad. Sci. USA 1987, 84, 2565–2569;
- 3bE. C. Constable, Tetrahedron 1992, 48, 10 013–10 059;
- 3cC. Piguet, G. Bernardinelli, B. Bocquet, A. Quattropanni, A. F. Williams, J. Am. Chem. Soc. 1992, 114, 7440–7451;
- 3dR. Krämer, J.-M. Lehn, A. De Cian, J. Fischer, Angew. Chem. 1993, 105, 764–767; Angew. Chem. Int. Ed. Engl. 1993, 32, 703–706;
- 3eR. F. Carina, G. Berunardinelli, A. F. Williams, Angew. Chem. 1993, 105, 1483–1485; Angew. Chem. Int. Ed. Engl. 1993, 32, 1463–1465;
- 3fE. C. Constable, A. J. Edwards, P. R. Raithby, J. V. Walker, Angew. Chem. 1993, 105, 1486–1488; Angew. Chem. Int. Ed. Engl. 1993, 32, 1465–1467;
- 3gN. Ohata, H. Masuda, O. Yamauchi, Angew. Chem. 1996, 108, 570–572;
10.1002/ange.19961080510 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 531–532.
- 4
- 4aK. Inoue, H. Imai, P. S. Ghalasasi, K. Kikuchi, M. Ohba, H. Ōkawa, J. V. Yakhmi, Angew. Chem. 2001, 113, 4372–4375; Angew. Chem. Int. Ed. 2001, 40, 4242–4245;
- 4bE. Coronado, C. J. Gómez-García, A. Nuez, F. M. Romero, E. Rusanov, H. Stoeckli-Evans, Inorg. Chem. 2002, 41, 4615–4617;
- 4cK. Inoue, K. Kikuchi, M. Ohba, H. Ōkawa, Angew. Chem. 2003, 115, 4958–4961; Angew. Chem. Int. Ed. 2003, 42, 4810–4813.
- 5Crystal data for L-isomer (L-1): Crystal size 0.35×0.35×0.20 mm3. C5H9Cr0.33MnN4O3, Mr=245.44, hexagonal, P63 (No. 173), a=b=13.6802(16), c=8.1530(14) Å, V=1321.4(3) Å3, T=100 K, Z=6, ρcalcd=1.851 g cm−3, μ(MoKα)=1.870 mm−1. Data were collected with a Bruker SMART-APEX three-circle diffractometer, equipped with a CCD area detector (graphite-monochromated MoKα radiation, λ=0.71073 Å, ω-scan mode (0.3 ° steps), semi-empirical absorption correction on Laue equivalents). The structures were solved by direct method and refined by full-matrix least squares against F2 of all data, using SHELXTL software. All-non-hydrogen atoms were refined anisotropically. Hydrogen atoms, except for the water hydrogen atoms, were placed in calculated positions but not refined. The refinement converges with R1=0.0440 for 1192 data (I>2σ(I)), wR2=0.1169 for 1194 unique data (1.72≥θ≥23.24 °), Flack parameter=0.05(6), max/min residual electron density 0.566/−0.608 e Å3. CCDC-238055 (L-1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 6See Supporting Information.