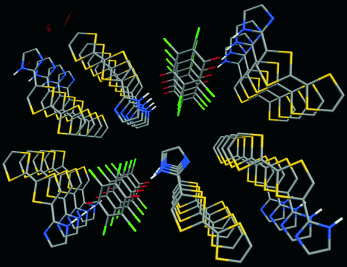
A Purely Organic Molecular Metal Based on a Hydrogen-Bonded Charge-Transfer Complex: Crystal Structure and Electronic Properties of TTF-Imidazole–p-Chloranil†
Tsuyoshi Murata
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan, Fax: (+81) 6-6850-5395
Search for more papers by this authorYasushi Morita Prof. Dr.
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan, Fax: (+81) 6-6850-5395
PRESTO, JST, Japan
Search for more papers by this authorKazunobu Sato Prof. Dr.
Departments of Chemistry and Materials Science, Graduate School of Science, Osaka City University, Sumiyoshi-ku, Osaka 558-8585, Japan
Search for more papers by this authorDaisuke Shiomi Prof. Dr.
Departments of Chemistry and Materials Science, Graduate School of Science, Osaka City University, Sumiyoshi-ku, Osaka 558-8585, Japan
Search for more papers by this authorTakeji Takui Prof. Dr.
Departments of Chemistry and Materials Science, Graduate School of Science, Osaka City University, Sumiyoshi-ku, Osaka 558-8585, Japan
Search for more papers by this authorMitsuhiko Maesato Prof. Dr.
Division of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan, Fax: (+81) 75-753-4035
Search for more papers by this authorHideki Yamochi Prof. Dr.
Research Center for Low Temperature and Materials Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan
Search for more papers by this authorGunzi Saito Prof. Dr.
Division of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan, Fax: (+81) 75-753-4035
Search for more papers by this authorKazuhiro Nakasuji Prof. Dr.
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan, Fax: (+81) 6-6850-5395
Search for more papers by this authorTsuyoshi Murata
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan, Fax: (+81) 6-6850-5395
Search for more papers by this authorYasushi Morita Prof. Dr.
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan, Fax: (+81) 6-6850-5395
PRESTO, JST, Japan
Search for more papers by this authorKazunobu Sato Prof. Dr.
Departments of Chemistry and Materials Science, Graduate School of Science, Osaka City University, Sumiyoshi-ku, Osaka 558-8585, Japan
Search for more papers by this authorDaisuke Shiomi Prof. Dr.
Departments of Chemistry and Materials Science, Graduate School of Science, Osaka City University, Sumiyoshi-ku, Osaka 558-8585, Japan
Search for more papers by this authorTakeji Takui Prof. Dr.
Departments of Chemistry and Materials Science, Graduate School of Science, Osaka City University, Sumiyoshi-ku, Osaka 558-8585, Japan
Search for more papers by this authorMitsuhiko Maesato Prof. Dr.
Division of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan, Fax: (+81) 75-753-4035
Search for more papers by this authorHideki Yamochi Prof. Dr.
Research Center for Low Temperature and Materials Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan
Search for more papers by this authorGunzi Saito Prof. Dr.
Division of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan, Fax: (+81) 75-753-4035
Search for more papers by this authorKazuhiro Nakasuji Prof. Dr.
Department of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan, Fax: (+81) 6-6850-5395
Search for more papers by this authorThis work was partially supported by PRESTO-JST, and by 21COE program “Creation of Integrated EcoChemistry of Osaka University”.
Graphical Abstract
Modification of tetrathiafulvalene with an imidazolyl substituent as a hydrogen-bond donor allows the generation of a hydrogen-bonded 2:1 charge-transfer complex with chloranil. This complex forms a three-dimensional network with columnar stacking of each component and multiple S⋅⋅⋅S interactions (see picture). This strongly hydrogen-bonded purely organic conducting material is the first to display metallic behavior.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z460801_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. R. Bryce, J. Mater. Chem. 1995, 5, 1481;
- 1bP. Batail, K. Boubekeur, M. Fourmigué, J.-C. P. Gabriel, Chem. Mater. 1998, 10, 3005.
- 2For a recent overview of H-bonding, see The Weak Hydrogen Bond (Eds.: ), Oxford University Press, New York, 1999, chap. 1.
- 3Examples of H-bonded TTF derivatives: Hydroxymethyl:
- 3aP. Blanchard, K. Boubekeur, M. Sallé, G. Duguay, M. Jubault, A. Gorgues, J. D. Martin, E. Canadell, P. Auban-Senzier, D. Jérome, P. Batail, Adv. Mater. 1992, 4, 579. Carboxy:
- 3bA. Dolbecq, M. Fourmigué, P. Batail, Bull. Soc. Chim. Fr. 1996, 133, 83; Amide:
- 3cK. Heuzé, C. Mézière, M. Fourmigué, P. Batail, C. Coulon, E. Canadell, P. Auban-Senzier, D. Jérome, Chem. Mater. 2000, 12, 1898;
- 3dK. Heuzé, M. Fourmigué, P. Batail, E. Canadell, P. Auban-Senzier, Chem. Eur. J. 1999, 5, 2971;
10.1002/(SICI)1521-3765(19991001)5:10<2971::AID-CHEM2971>3.0.CO;2-S CAS Web of Science® Google Scholar
- 3eS. A. Baudron, N. Avarvari, P. Batail, C. Coulon, R. Clérac, E. Canadell, P. Auban-Senzier, J. Am. Chem. Soc. 2003, 125, 11 583. Thioamide:
- 3fA. J. Moore, M. R. Bryce, A. S. Batsanov, J. N. Heaton, C. W. Lehmann, J. A. K. Howard, N. Robertson, A. E. Underhill, I. F. Perepichka, J. Mater. Chem. 1998, 8, 1541. Nucleic acid:
- 3gY. Morita, S. Maki, M. Ohmoto, H. Kitagawa, T. Okubo, T. Mitani, K. Nakasuji, Org. Lett. 2002, 4, 2185. Pyrrole-fused:
- 3hK. Zong, W. Chen, M. P. Cava, R. D. Rogers, J. Org. Chem. 1996, 61, 8117;
- 3iJ. O. Jeppesen, K. Takimiya, F. Jensen, T. Brimert, K. Nielsen, N. Thorup, J. Becher, J. Org. Chem. 2000, 65, 5794;
- 3jK. A. Nielsen, J. O. Jeppesen, E. Levillain, J. Becher, Angew. Chem. 2003, 115, 197;
10.1002/ange.200390042 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 187; for a review, see:
- 3kJ. O. Jeppesen, J. Becher, Eur. J. Org. Chem. 2003, 3245. Uracil-fused:
- 3lO. Neilands, S. Belyakov, V. Tilika, A. Edzina, J. Chem. Soc. Chem. Commun. 1995, 325;
- 3mO. Neilands, V. Liepinsh, B. Turovska, Org. Lett. 1999, 1, 2065;
- 3nK. Balodis, S. Khasanov, C. Chong, M. Maesato, H. Yamochi, G. Saito, O. Neilands, Synth. Met. 2003, 133–134, 353.
- 4
- 4aT. Akutagawa, G. Saito, Bull. Chem. Soc. Jpn. 1995, 68, 1753;
- 4bT. Akutagawa, G. Saito, M. Kusunoki, K. Sakaguchi, Bull. Chem. Soc. Jpn. 1996, 69, 2487.
- 5
- 5aM. Tadokoro, H. Kanno, T. Kitajima, H. S. Umemoto, N. Nakanishi, K. Isobe, K. Nakasuji, Proc. Natl. Acad. Sci. USA 2002, 99, 4950;
- 5bY. Morita, T. Murata, K. Fukui, M. Tadokoro, K. Sato, D. Shiomi, T. Takui, K. Nakasuji, Chem. Lett. 2004, 33, 188.
- 6Y. Morita, T. Murata, H. Yamochi, G. Saito, K. Nakasuji, Synth. Met. 2003, 135–136, 579. See also the Supporting Information.
- 7TTF-CHL is a neutral CT complex with 1:1 component ratio and is a semiconductor with low electrical conductivity (σrt=∼10−4 S cm−1): J. B. Torrance, J. J. Mayerle, V. Y. Lee, K. Bechgaard, J. Am. Chem. Soc. 1979, 101, 4747.
- 8Crystal data for TTF-Im: C9H6N2S4, Mr=270.40, crystal dimensions 0.25×0.15×0.05 mm3, orthorhombic, space group Pna21 (no. 33), a=10.198(1), b=25.196(4), c=4.1681(5) Å, V=1071.0(2) Å3, Z=4, ρcalcd=1.677 g cm−3, μ=8.49 cm−1, 2 θmax=55.0°, λ(MoKα)=0.71075 Å, ω scan mode, T=200 K, 9749 reflections, of which 2853 were unique and 2116 were included in the refinement [I>2.00 σ(I)], data corrected for Lorentzian and polarization effects; an empirical absorption correction resulted in transmission factors ranging from 0.8302 to 0.9582, solution by direct methods (MITHRIL 90) and refinement on |F2| by full-matrix least-squares procedures (SHELXL97), 136 parameters, the non-H atoms were refined anisotropically, H atoms were included but not refined, final values R1=0.032, wR2=0.069, GOF=1.06, maximum positive and negative peaks in ΔF map were ρmax=0.26 e Å−3 and ρmin=−0.21 e Å−3. CCDC-239640 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 9
- 9aS. Martinez-Carrera, Acta Crystallogr. 1966, 20, 783;
- 9bB. M. Craven, R. K. McMullan, J. D. Bell, H. C. Freeman, Acta Crystallogr. Sect. B 1977, 33, 2585.
- 10Crystal data for (TTF-Im)2(CHL): C12H6N2OS4Cl2, Mr=393.34, crystal dimensions 0.30×0.05×0.02 mm3, triclinic, space group P
 (no. 2), a=3.7573(2), b=12.2435(4), c=15.8037(5) Å, α=93.996(3), β=85.915(3), χ=82.138(3)°, V=716.02(5) Å3, Z=2, ρcalcd=1.824 g cm−3, μ=95.20 cm−1, 2 θmax=55.0°, λ(CuKα)=1.54178 Å, ω scan mode, T=293 K, 8786 reflections, of which 2521 were unique and 7895 were included in the refinement [I>2.00 σ(I)], data corrected for Lorentzian and polarization effects; an empirical absorption correction resulted in transmission factors ranging from 0.7196 to 1.0000, solutions by direct methods (SIR 97) and refinement on |F2| by full-matrix least-squares procedures, 192 parameters, the non-H atoms were refined anisotropically, H atoms were included but not refined, final values R1=0.058, wR2=0.171, GOF=1.068, maximum positive and negative peaks in ΔF map were ρmax=0.42 e Å−3 and ρmin=−0.42 e Å−3. CCDC 239641 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
(no. 2), a=3.7573(2), b=12.2435(4), c=15.8037(5) Å, α=93.996(3), β=85.915(3), χ=82.138(3)°, V=716.02(5) Å3, Z=2, ρcalcd=1.824 g cm−3, μ=95.20 cm−1, 2 θmax=55.0°, λ(CuKα)=1.54178 Å, ω scan mode, T=293 K, 8786 reflections, of which 2521 were unique and 7895 were included in the refinement [I>2.00 σ(I)], data corrected for Lorentzian and polarization effects; an empirical absorption correction resulted in transmission factors ranging from 0.7196 to 1.0000, solutions by direct methods (SIR 97) and refinement on |F2| by full-matrix least-squares procedures, 192 parameters, the non-H atoms were refined anisotropically, H atoms were included but not refined, final values R1=0.058, wR2=0.171, GOF=1.068, maximum positive and negative peaks in ΔF map were ρmax=0.42 e Å−3 and ρmin=−0.42 e Å−3. CCDC 239641 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 11Selected physical data: (TTF-Im)2(CHL), IR (KBr):
 =3200–2700, 1532 cm−1; UV/Vis (KBr): λmax=290, 386, 700, 834 nm; elemental analysis (%): calcd for (C9H6N2S4)2(C6O2Cl4): C 36.64, H 1.54, N 7.12; found: C 36.77, H 1.57, N 7.22.
=3200–2700, 1532 cm−1; UV/Vis (KBr): λmax=290, 386, 700, 834 nm; elemental analysis (%): calcd for (C9H6N2S4)2(C6O2Cl4): C 36.64, H 1.54, N 7.12; found: C 36.77, H 1.57, N 7.22.
- 12A. Girlando, I. Zanon, R. Bozio, C. Pecile, J. Chem. Phys. 1978, 68, 22.
- 13(TTF-Im)2(CHL) shows the lower-energy absorption band at around 3000 cm−1, which is characteristic of partial CT complexes with segregated stacking columns (see the Supporting Information).
- 14The oxidation potential of TTF-based donor molecules with an amido moiety is strongly affected by formation of H-bonds to inorganic anions.[3c]
- 15Calculation method: T. Mori, A. Kobayashi, Y. Sasaki, H. Kobayashi, G. Saito, H. Inokuchi, Bull. Chem. Soc. Jpn. 1984, 57, 627.
- 16The amount of the temperature-dependent contribution, 1.1 %, was determined by the saturation magnetization at 1.9 K (123.01 erg Oe−1 mol−1) under the assumption that a unit of (TTF-Im)2(CHL) has nominally two S=1/2 spins by complexation.
- 17The ESR spectra observed at all temperatures were reproduced by spectral simulation for a randomly oriented system, which assumes temperature-independent g anisotropy (see text) and temperature-dependent peak-to-peak linewidths (inset of Figure 5).
- 18The 2 J value was calculated at the UB3LYP/6-31G(d) level according to the procedure developed by Yamaguchi et al.: Y. Takano, T. Taniguchi, H. Isobe, T. Kubo, Y. Morita, K. Yamamoto, K. Nakasuji, T. Takui, K. Yamaguchi, J. Am. Chem. Soc. 2002, 124, 11 122, and references therein.
- 19J. C. Bonner, M. E. Fisher, Phys. Rev. A 1964, 135, 640.
- 20R. P. Groff, A. Suna, R. E. Merrifield, Phys. Rev. Lett. 1974, 33, 418.
- 21A phase transition in H-bonded CT complex: K. Nakasuji, K. Sugiura, T. Kitagawa, J. Toyoda, H. Okamoto, K. Okaniwa, T. Mitani, H. Yamamoto, I. Murata, A. Kawamoto, J. Tanaka, J. Am. Chem. Soc. 1991, 113, 1862.