Pd-Catalyzed Amination of Nucleoside Arylsulfonates to yield N6-Aryl-2,6-Diaminopurine Nucleosides†
Padmaja Gunda
Department of Chemistry, The City College and The City University of New York, 138th Street at Convent Avenue, New York, NY 10031, USA, Fax: (+1) 212-650-6107
Search for more papers by this authorLarry M. Russon Dr.
Analytica of Branford Inc. 29 Business Park Drive, Branford, CT 06405, USA
Search for more papers by this authorMahesh K. Lakshman Prof.
Department of Chemistry, The City College and The City University of New York, 138th Street at Convent Avenue, New York, NY 10031, USA, Fax: (+1) 212-650-6107
Search for more papers by this authorPadmaja Gunda
Department of Chemistry, The City College and The City University of New York, 138th Street at Convent Avenue, New York, NY 10031, USA, Fax: (+1) 212-650-6107
Search for more papers by this authorLarry M. Russon Dr.
Analytica of Branford Inc. 29 Business Park Drive, Branford, CT 06405, USA
Search for more papers by this authorMahesh K. Lakshman Prof.
Department of Chemistry, The City College and The City University of New York, 138th Street at Convent Avenue, New York, NY 10031, USA, Fax: (+1) 212-650-6107
Search for more papers by this authorThis work was supported by NSF grant CHE-0314326 and a PSC-CUNY 35 award. Acquisition of a 500 MHz NMR spectrometer was funded by NSF grant CHE-0210295. Professor S. L. Buchwald (MIT) is thanked for a sample of L 5, and Dr. G. Li (CombiPhos Catalysts Inc.) is thanked for samples of L 6 and L 7.
Graphical Abstract
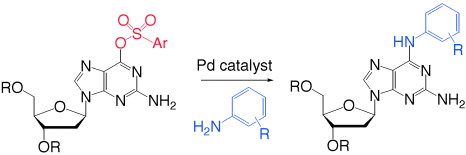
Substituents on both the nucleoside arylsulfonate as well as the aryl amine component have a significant impact on their coupling to form 2,6-diaminopurine-2′-deoxyribonucleosides (see scheme). A systematic study of ligands for the Pd catalysts in amination and CC cross-coupling reactions gives insight into the structural elements that lead to effective catalysis.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z460782_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews on this topic, see:
- 1aJ. P. Wolfe, S. Wagaw, J.-F. Marcoux, S. L. Buchwald, Acc. Chem. Res. 1999, 31, 805–818;
- 1bJ. F. Hartwig, Acc. Chem. Res. 1999, 31, 852–860;
- 1cC. G. Frost, P. Mendonca, J. Chem. Soc. Perkin Trans. 1 1998, 2615–2623;
- 1dB. H. Yang, S. L. Buchwald, J. Organomet. Chem. 1999, 576, 125–146;
- 1eJ. F. Hartwig, in Modern Amination Methods, (Ed.: A. Ricci), Wiley-VCH, Weinheim, 2000, pp. 195–262;
10.1002/9783527613182.ch7 Google Scholar
- 1fA. R. Muci, S. L. Buchwald, Top. Curr. Chem. 2002, 219, 131–209;
- 1gD. Prim, J.-M. Campagne, D. Joseph, B. Andrioletti, Tetrahedron 2002, 58, 2041–2075.
- 2
- 2aM. K. Lakshman, J. C. Keeler, J. H. Hilmer, J. Q. Martin, J. Am. Chem. Soc. 1999, 121, 6090–6091;
- 2bM. K. Lakshman, J. H. Hilmer, J. Q. Martin, J. C. Keeler, Y. Q. V. Dinh, F. N. Ngassa, L. M. Russon, J. Am. Chem. Soc. 2001, 123, 7779–7787;
- 2cM. K. Lakshman, P. Gunda, Org. Lett. 2003, 5, 39–42;
- 2dM. K. Lakshman, F. N. Ngassa, S. Bae, D. G. Buchanan, H.-G. Hahn, H. Mah, J. Org. Chem. 2003, 68, 6020–6030.
- 3
- 3aE. A. Harwood, S. T. Sigurdsson, N. B. F. Edfeldt, B. R. Reid, P. B. Hopkins, J. Am. Chem. Soc. 1999, 121, 5081–5082;
- 3bF. De Riccardis, R. R. Bonala, F. Johnson, J. Am. Chem. Soc. 1999, 121, 10 453–10 460;
- 3cE. A. Harwood, P. B. Hopkins, S. T. Sigurdsson, J. Org. Chem. 2000, 65, 2959–2964;
- 3dR. Bonala, I. Shishkina, F. Johnson, Tetrahedron Lett. 2000, 41, 7281–7284;
- 3eF. De Riccardis, F. Johnson, Org. Lett. 2000, 3, 293–295;
- 3fZ. Wang, C. J. Rizzo, Org. Lett. 2001, 3, 565–568;
- 3gE. Schoffers, P. D. Olsen, J. C. Means, Org. Lett. 2001, 3, 4221–4223;
- 3hC. Meier, S. Gräsl, Synlett 2002, 802–804;
- 3iF. Johnson, R. Bonala, D. Tawde, M. C. Torres, C. R. Iden, Chem. Res. Toxicol. 2002, 15, 1489–1494;
- 3jL. C. Gillet, O. D. Schärer, Org. Lett. 2002, 4, 4205–4208;
- 3kD. Chakraborti, L. Colis, R. Schneider, A. K. Basu, Org. Lett. 2003, 5, 2861–2864;
- 3lT. Takamura-Enya, S. Ishikawa, M. Mochizuki, K. Wakabayashi, Tetrahedron Lett. 2003, 44, 5969–5973.
- 4For reviews on Pd-catalyzed CN bond-forming reactions of nucleosides, see:
- 4aM. K. Lakshman, J. Organomet. Chem. 2002, 653, 234–251;
- 4bM. K. Lakshman, Curr. Org. Synth. 2005, in press.
- 5
- 5aH. P. Daskalov, M. Sekine, T. Hata, Tetrahedron Lett. 1980, 21, 3899–3902;
- 5bB. L. Gaffney, R. A. Jones, Tetrahedron Lett. 1982, 23, 2253–2256;
- 5cY. Hayakawa, M. Hirose, R. Noyori, J. Org. Chem. 1993, 58, 5551–5555;
- 5dC. B. Reese, P. A. Skone, J. Chem. Soc. Perkin Trans. 1 1984, 1263–1271.
- 6aP. K. Bridson, W. T. Markiewicz, C. B. Reese, J. Chem. Soc. Chem. Commun. 1977, 791–792;
- 6bY.-Z. Xu, Q. Zheng, P. F. Swann, Tetrahedron 1991, 32, 2817–2820;
- 6cC. R. Allerson, S. L. Chen, G. L. Verdine, J. Am. Chem. Soc. 1997, 119, 7423–7433.
- 7M. K. Lakshman, F. N. Ngassa, J. C. Keeler, Y. Q. V. Dinh, J. H. Hilmer, L. M. Russon, Org. Lett. 2000, 2, 927–930.
- 8E. A. Véliz, P. A. Beal, J. Org. Chem. 2001, 66, 8592–8598.
- 9X. Huang, K. W. Anderson, D. Zim, L. Jiang, A. Klapars, S. L. Buchwald, J. Am. Chem. Soc. 2003, 125, 6653–6655.
- 10A. H. Roy, J. F. Hartwig, J. Am. Chem. Soc. 2003, 125, 8704–8705.
- 11M. K. Lakshman, P. F. Thomson, M. A. Nuqui, J. H. Hilmer, N. Sevova, B. Boggess, Org. Lett. 2002, 4, 1479–1482.
- 12Ligands L 1 to L 5 are commercially available from Strem Chemicals, whereas L 6 and L 7 are available from CombiPhos Catalysts Inc.
- 13
- 13aP. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek, Acc. Chem. Res. 2001, 34, 895–904;
- 13bJ. Yin, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 6043–6048;
- 13cI. del Rio, N. Ruiz, C. Claver, L. van der Veen, P. W. N. M. van Leeuwen, J. Mol. Catal. 2000, 161, 39–48.
- 14Amination reactions of 3 a were also conducted with 2-anisidine and 4-aminoacetophenone in the presence of Pd(OAc)2 (10 mol %) and L 4 (30 mol %) in toluene at 110 °C. The progress of these reactions was observed with product recoveries of 50 % (reaction time: 1.75 h) and 25 % (reaction time: 3 h), respectively.
- 15
- 15aM. Hocek, A. Holý, I. Votruba, H. Dvořaková, J. Med. Chem. 2000, 43, 1817–1825;
- 15bM. Hocek, A. Holý, I. Votruba, H. Dvořaková, Collect. Czech. Chem. Commun. 2000, 65, 1683–1697;
- 15cM. Hocek, A. Holý, I. Votruba, H. Dvořaková, Collect. Czech. Chem. Commun. 2001, 66, 483–499.
- 16
- 16aM. Havelkova, M. Hocek, M. Česnek, D. Dvořak, Synlett 1999, 1145–1147;
- 16bM. Havelkova, D. Dvořak, M. Hocek, Synlett 2001, 1704–1710.
- 17S. M. Reid, R. C. Boyle, J. T. Mague, M. J. Fink, J. Am. Chem. Soc. 2003, 125, 7816–7817.
- 18D. Zim, S. L. Buchwald, Org. Lett. 2003, 14, 2413–2415.