Asymmetric Synthesis of Highly Substituted β-Lactones by Nucleophile-Catalyzed [2+2] Cycloadditions of Disubstituted Ketenes with Aldehydes†
Jonathan E. Wilson
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-324-3611
Search for more papers by this authorGregory C. Fu Prof. Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-324-3611
Search for more papers by this authorJonathan E. Wilson
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-324-3611
Search for more papers by this authorGregory C. Fu Prof. Dr.
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA, Fax: (+1) 617-324-3611
Search for more papers by this authorWe thank Ivory D. Hills for X-ray crystallographic studies. Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences: R01-GM57034; National Cancer Institute: training grant CA009112), Merck, and Novartis. Funding for the MIT Department of Chemistry Instrumentation Facility has been furnished in part by NSF CHE-9808061 and NSF DBI-9729592.
Graphical Abstract
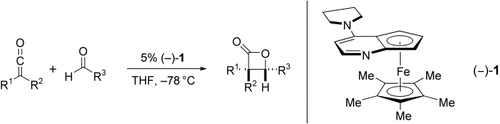
α,α-Disubstituted β-lactones can be obtained by the cycloaddition of the corresponding ketenes with aldehydes (see scheme). For the first time, a chiral PPY derivative, 1, serves as an efficient catalyst for the asymmetric synthesis of β-lactones (PPY=4-pyrrolidin-1-ylpyridine). To date, this is the only catalyst that is effective for enantioselective cycloadditions of disubstituted ketenes with aldehydes.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z460698_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For a review of the synthesis of optically active β-lactones, see: H. W. Yang, D. Romo, Tetrahedron 1999, 55, 6403–6434.
- 2For reviews of naturally occurring β-lactones, see:
- 2aC. Lowe, J. C. Vederas, Org. Prep. Proced. Int. 1995, 27, 305–346;
- 2bA. Pommier, J.-M. Pons, Synthesis 1995, 729–744.
- 3For studies of analogues of lactacystin β-lactone (and leading references), see:
- 3aE. J. Corey, W. Li, G. A. Reichard, J. Am. Chem. Soc. 1998, 120, 2330–2336;
- 3bF. Soucy, L. Grenier, M. L. Behnke, A. T. Destree, T. A. McCormack, J. Adams, L. Plamondon, J. Am. Chem. Soc. 1999, 121, 9967–9976.
- 4For an example of an application in material science, see: M. E. Gelbin, J. Kohn, J. Am. Chem. Soc. 1992, 114, 3962–3965.
- 5S. G. Nelson, W. S. Cheung, A. J. Kassick, M. A. Hilfiker, J. Am. Chem. Soc. 2002, 124, 13 654–13 655.
- 6Z. Wan, S. G. Nelson, J. Am. Chem. Soc. 2000, 122, 10 470–10 471.
- 7J. Taunton, J. L. Collins, S. L. Schreiber, J. Am. Chem. Soc. 1996, 118, 10 412–10 422.
- 8For leading references to methods for the catalytic asymmetric synthesis of β-lactones, see: C. Schneider, Angew. Chem. 2002, 114, 771–772;
10.1002/1521-3757(20020301)114:5<771::AID-ANGE771>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2002, 41, 744–746.10.1002/1521-3773(20020301)41:5<744::AID-ANIE744>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 9For industrial applications of catalytic asymmetric [2+2] cycloadditions of ketenes with aldehydes, see: P. Stutte in Chirality in Industry (Eds.: ), Wiley, New York, 1997, chap. 18.
- 10For chiral nucleophilic catalysts, see:
- 10aD. Borrmann, R. Wegler, Chem. Ber. 1966, 99, 1245–1251; D. Borrmann, R. Wegler, Chem. Ber. 1966, 99, 1575–1579;
- 10bH. Wynberg, E. G. J. Staring, J. Am. Chem. Soc. 1982, 104, 166–168; H. Wynberg, E. G. J. Staring, J. Org. Chem. 1985, 50, 1977–1979; P. E. F. Ketelaar, H. Wynberg, E. G. J. Staring, Tetrahedron Lett. 1985, 26, 4665–4668;
- 10cR. Tennyson, D. Romo, J. Org. Chem. 2000, 65, 7248–7252; G. S. Cortez, R. L. Tennyson, D. Romo, J. Am. Chem. Soc. 2001, 123, 7945–7946; G. S. Cortez, S. H. Oh, D. Romo, Synthesis 2001, 1731–1736;
- 10dC. Zhu, X. Shen, S. G. Nelson, J. Am. Chem. Soc. 2004, 126, 5352–5353.
- 11Chiral Lewis acid catalysts:
- 11afor an overview, see: ref. [8];
- 11bfor leading references, see: S. G. Nelson, C. Zhu, X. Shen, J. Am. Chem. Soc. 2004, 126, 14–15.
- 12Salinosporamide A and omuralide are two examples of natural products that include an α,α-disubstituted β-lactone. For leading references, see: L. R. Reddy, P. Saravanan, E. J. Corey, J. Am. Chem. Soc. 2004, 126, 6230–6231.
- 13
- 13aFor an early overview, see: G. C. Fu, Acc. Chem. Res. 2000, 33, 412–420;
- 13bFor more recent reports, see: A. H. Mermerian, G. C. Fu, J. Am. Chem. Soc. 2003, 125, 4050–4051;
I. D. Hills, G. C. Fu, Angew. Chem. 2003, 115, 4051–4054;
10.1002/ange.200351666 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3921–3924.
- 14B. L. Hodous, G. C. Fu, J. Am. Chem. Soc. 2002, 124, 1578–1579.
- 15A. E. Taggi, A. M. Hafez, H. Wack, B. Young, W. J. Drury III, T. Lectka, J. Am. Chem. Soc. 2000, 122, 7831–7832.
- 16See ref. [11b]. Examples of cinchona alkaloid-based intramolecular reactions of monosubstituted ketenes had been described earlier (ref. [10c]).
- 17For reviews of catalytic asymmetric methods that generate quaternary stereocenters, see: E. J. Corey, A. Guzman-Perez, Angew. Chem. 1998, 110, 403–415;
Angew. Chem. Int. Ed. 1998, 37, 388–401;
10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google ScholarJ. Christoffers, A. Mann, Angew. Chem. 2001, 113, 4725–4732;10.1002/1521-3757(20011217)113:24<4725::AID-ANGE4725>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4591–4597; see also:10.1002/1521-3773(20011217)40:24<4591::AID-ANIE4591>3.0.CO;2-V CAS PubMed Web of Science® Google ScholarI. Denissova, L. Barriault, Tetrahedron 2003, 59, 10 105–10 146.
- 18Notes:
- 18aUnder our standard conditions (Table 2), aryl alkyl ketenes, monosubstituted ketenes, very electron-rich aldehydes, and non-aromatic aldehydes are not suitable substrates.
- 18bAt the end of a reaction, we can typically recover about 80 % of catalyst 1.
- 19A. Griesbeck, D. Seebach, Helv. Chim. Acta 1987, 70, 1326–1332. For a more recent study, see:
S. G. Nelson, K. L. Spencer, Angew. Chem. 2000, 112, 1379–1381;
Angew. Chem. Int. Ed. 2000, 39, 1323–1325.
10.1002/(SICI)1521-3773(20000403)39:7<1323::AID-ANIE1323>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 20For a review of the enantioselective synthesis of β-amino acids, see:
- 20aE. Juaristi, Enantioselective Synthesis of β-Amino Acids, Wiley-VCH, New York, 1997;
- 20bM. Liu, M. P. Sibi, Tetrahedron 2002, 58, 7991–8035.
- 21In preliminary experiments, we have not observed ring-opening upon reaction with RSH, R2NH, or R2NLi.