A Cyclobutadiene Equivalent in the Catalytic Pauson–Khand Reaction†
Susan E. Gibson Prof.
Department of Chemistry, Imperial College London, South Kensington Campus, London SW7 2AY, UK, Fax: (+44) 207-594-5804
Search for more papers by this authorNello Mainolfi
Department of Chemistry, Imperial College London, South Kensington Campus, London SW7 2AY, UK, Fax: (+44) 207-594-5804
Search for more papers by this authorS. Barret Kalindjian Dr.
James Black Foundation, 68 Half Moon Lane, London SE24 9JE, UK
Search for more papers by this authorPaul T. Wright Dr.
James Black Foundation, 68 Half Moon Lane, London SE24 9JE, UK
Search for more papers by this authorSusan E. Gibson Prof.
Department of Chemistry, Imperial College London, South Kensington Campus, London SW7 2AY, UK, Fax: (+44) 207-594-5804
Search for more papers by this authorNello Mainolfi
Department of Chemistry, Imperial College London, South Kensington Campus, London SW7 2AY, UK, Fax: (+44) 207-594-5804
Search for more papers by this authorS. Barret Kalindjian Dr.
James Black Foundation, 68 Half Moon Lane, London SE24 9JE, UK
Search for more papers by this authorPaul T. Wright Dr.
James Black Foundation, 68 Half Moon Lane, London SE24 9JE, UK
Search for more papers by this authorThe authors thank the James Black Foundation for generous studentship support (N.M.).
Graphical Abstract
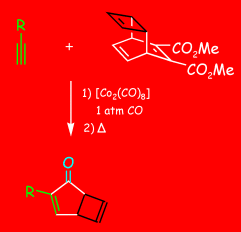
A practical and scalable operation: The reaction shown in the scheme, which uses catalytic amounts of hexacarbonyldicobalt, gives access to versatile bicyclic systems, which until now could only be obtained in low quantities by a photochemical process starting from tropolones. An isolation of the primary Pauson–Khand products is not necessary.
References
- 1For reviews of the PKR, see:
- 1aS. E. Gibson, A. Stevenazzi, Angew. Chem. 2003, 115, 1844–1854;
10.1002/ange.200200547 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1800–1810;
- 1bB. E. Hanson, Comments Inorg. Chem. 2002, 23, 289–318;
- 1cA. J. Fletcher, S. D. R. Christie, J. Chem. Soc. Perkin Trans. 1 2000, 1657–1668;
- 1dK. M. Brummond, J. L. Kent, Tetrahedron 2000, 56, 3263–3283;
- 1eY. K. Chung, Coord. Chem. Rev. 1999, 188, 297–341;
- 1fO. Geis, H.-G. Schmalz, Angew. Chem. 1998, 110, 955–958;
10.1002/(SICI)1521-3757(19980403)110:7<955::AID-ANGE955>3.0.CO;2-T Google ScholarAngew. Chem. Int. Ed. 1998, 37, 911–914.10.1002/(SICI)1521-3773(19980420)37:7<911::AID-ANIE911>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 2J. Marrero, A. D. Rodriguez, P. Baran, R. G. Raptis, J. A. Sanchez, E. Ortega-Barria, T. L. Capson, Org. Lett. 2004, 6, 1661–1664.
- 3S. M. Roberts, M. G. Santoro, E. S. Sickle, J. Chem. Soc. Perkin Trans. 1 2002, 1735–1742.
- 4aI. Marchueta, X. Verdaguer, A. Moyano, M. A. Pericas, A. Riera, Org. Lett. 2001, 3, 3193–3196;
- 4bP. Bladon, I. U. Khand, P. L. Pauson, J. Chem. Res. Synop. 1977, 8;
- 4cV. Sampath, E. C. Lund, M. J. Knudsen, M. M. Olmstead, N. E. Schore, J. Org. Chem. 1987, 52, 3595–3603;
- 4dB. A. Kowalczyk, T. C. Smith, W. G. Dauben, J. Org. Chem. 1998, 63, 1379–1389.
- 5aM. R. Rivero, J. C. de la Rosa, J. C. Carretero, J. Am. Chem. Soc. 2003, 125, 14 992–14 993;
- 5bK. Itami, K. Mitsudo, J.-I. Yoshida, Angew. Chem. 2002, 114, 3631–3634;
10.1002/1521-3757(20020916)114:18<3631::AID-ANGE3631>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3481–3483;10.1002/1521-3773(20020916)41:18<3481::AID-ANIE3481>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 5cP. A. Wender, N. M. Deschamps, T. J. Williams, Angew. Chem. 2004, 116, 3138–3141;
10.1002/ange.200454117 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3076–3079.
- 6
- 6aS. E. Gibson, C. Johnstone, J. A. Loch, J. W. Steed, A. Stevenazzi, Organometallics 2003, 22, 5374–5377;
- 6bA. C. Comely, S. E. Gibson, A. Stevenazzi, N. J. Hales, Tetrahedron Lett. 2001, 42, 1183–1185;
- 6cA. C. Comely, S. E. Gibson, N. J. Hales, Chem. Commun. 2000, 305–306.
- 7S. E. Gibson, S. E. Lewis, J. A. Loch, J. W. Steed, M. J. Tozer, Organometallics 2003, 22, 5382–5384.
- 8
- 8aE. E. van Tamelen, B. C. T. Pappas, J. Am. Chem. Soc. 1971, 93, 6111–6120;
- 8bA. G. Anderson, D. R. Fagerburg, Tetrahedron 1973, 29, 2973–2979;
- 8cW. G. Dauben, L. N. Reitman, J. Org. Chem. 1975, 40, 835–841.
- 9
- 9aI. D. Reingold, K. S. Kwong, M. M. Menard, J. Org. Chem. 1989, 54, 708–710;
- 9bT. Miyashi, M. Nitta, T. Mukai, J. Am. Chem. Soc. 1971, 93, 3441–3447;
- 9cM. Cavazza, M. Zandomeneghi, F. Pietra, J. Chem. Soc. Chem. Commun. 1990, 1336–1337.
- 10
- 10aK. V. Ivin, J. C. Mol, Olefin Metathesis and Metathesis Polymerization, Academic Press, San Diego, 1997, Chap. 11, pp. 224–259;
10.1016/B978-012377045-5/50012-4 Google Scholar
- 10b“Bioactive Polymers”: L. L. Kiessling, L. E. Strong in Alkene Metathesis in Organic Synthesis (Ed.: ), Springer, Berlin, 1998, pp. 199–231.
10.1007/3-540-69708-X_8 Google Scholar