Endohedral Zintl Ions: Intermetalloid Clusters
Graphical Abstract
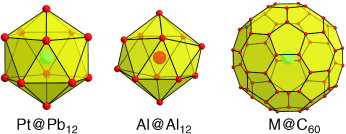
The close race between Zintl ions and fullerides has gone into the second round, initialized by the synthesis of the soluble endohedral cluster [Pt@Pb12]2− (see picture, left). The structural characterization of an anion with almost ideal icosahedral symmetry illustrates that many heteroatomic clusters observed in the gas phase can, indeed, be regarded as endohedral clusters.
Abstract
Tetrels can be regarded as most promising candidates for the construction of larger clusters. Recent examples have shown that larger clusters are particularly stable if they contain interstitial atoms (e.g. [Pt@Pb12]2−). Many salts of the polyhedral anions are soluble, but a number of examples—usually those with higher charges—occur only as quasi-discrete units in saltlike crystals (Zintl phases) or as building blocks in intermetallic phases. In this Minireview, the chemistry of intermetalloid clusters is reviewed with reference to the endohedral Zintl ions, Zintl phases, and polyhedral building blocks of intermetallic compounds, including heteroatomic species in the gas phase. We focus on selected examples and discuss the new findings in the context of recent advances in the field of metalloid clusters and (endohedral) fullerenes and fullerides.