Synthesis and Manipulation of Orthogonally Protected Dendrimers: Building Blocks for Library Synthesis†
Mackay B. Steffensen
Texas A&M University, Department of Chemistry, College Station, TX 77843-3255, USA, Fax: (+1) 979-845-9452
Search for more papers by this authorEric E. Simanek Dr.
Texas A&M University, Department of Chemistry, College Station, TX 77843-3255, USA, Fax: (+1) 979-845-9452
Search for more papers by this authorMackay B. Steffensen
Texas A&M University, Department of Chemistry, College Station, TX 77843-3255, USA, Fax: (+1) 979-845-9452
Search for more papers by this authorEric E. Simanek Dr.
Texas A&M University, Department of Chemistry, College Station, TX 77843-3255, USA, Fax: (+1) 979-845-9452
Search for more papers by this authorThis work was supported by a grant from the N.I.H. (NIGMS 64650). M.B.S. received a predoctoral fellowship from the Chemistry-Biology Training Grant (NIH TM GM08523).
Graphical Abstract
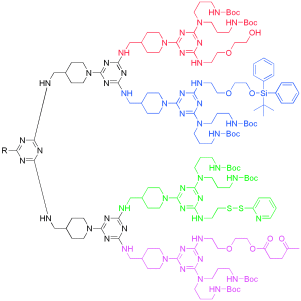
Fruits of the laboratory: Dendrimers containing up to six different functional groups can be prepared in multistep one-pot reactions by using a selective manipulation and deprotection strategy (see example). The authors call these multifunctional dendrimers molecular fruit-salad trees; they could function as useful building blocks for drug-delivery systems and novel materials.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z460031_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. M. Grayson, J. M. J. Fréchet, Chem. Rev. 2001, 101, 3819–3867;
- 1bG. R. Newkome, F. Vögtle, C. N. Moorefield, Dendrimers and Dendrons: Concepts, Syntheses, Applications, VCH, New York, 2001;
10.1002/3527600612 Google Scholar
- 1cJ. M. J. Frèchet, D. A. Tomalia, Dendrimers and Other Dendritic Polymers, Wiley, New York, 2002.
- 2
- 2aM. Okaniwa, K. Takeuchi, M. Asai, M. Ueda, Macromolecules 2002, 35, 6232–6238;
- 2bS. P. Rannard, N. J. Davis, J. Am. Chem. Soc. 2000, 122, 11 729—11 770.
- 3
- 3aP. Singh, Bioconjugate Chem. 1998, 9, 54–63;
- 3bG. R. Newkome, C. D. Weis, C. N. Moorefield, G. R. Baker, B. J. Childs, J. Epperson, Angew. Chem. 1998, 110, 318–321;
10.1002/(SICI)1521-3757(19980202)110:3<318::AID-ANGE318>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 1998, 37, 307–310;10.1002/(SICI)1521-3773(19980216)37:3<307::AID-ANIE307>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 3cG. R. Newkome, B. J. Childs, M. J. Rourk, G. R. Baker, C. N. Moorefield, Biotechnol. Bioeng. 1999, 61, 243–253;
- 3dJ. R. Baker, A. Quintana, L. Piehler, M. Banazak-Holl, D. Tomalia, E. Raczka, Biomed. Microdevices 2001, 3, 61–69.
- 4
- 4aW. Zhang, D. T. Nowlan III, L. M. Thomson, W. M. Lackowski, E. E. Simanek, J. Am. Chem. Soc. 2001, 123, 8914–8922;
- 4bZ. Bo, A. Schäfer, P. Franke, A. D. Schlüter, Org. Lett. 2000, 2, 1645–1648;
- 4cH.-F. Chow, C.-F. Leung, K.-W. Wong, L. Xi, Angew. Chem. 2003, 115, 5069–5073;
10.1002/ange.200351613 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4919–4923;
- 4dS. Li, M. L. Szalai, R. M. Kevwitch, D. V. McGrath, J. Am. Chem. Soc. 2003, 125, 10516–10517;
- 4eK. L. Wooley, C. J. Hawker, J. M. J. Fréchet, J. Chem. Soc. Perkin Trans. 1 1991, 1059–1076;
- 4fW. Zhang, S. E. Tichy, L. M. Pérez, G. Maria, P. A. Lindahl, E. E. Simanek, J. Am. Chem. Soc. 2003, 125, 5086–5094.
- 5
- 5aS.-E. Stiriba, H. Frey, R. Haag, Angew. Chem. 2002, 114, 1385–1390;
10.1002/1521-3757(20020415)114:8<1385::AID-ANGE1385>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1329–1334;10.1002/1521-3773(20020415)41:8<1329::AID-ANIE1329>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 5bF. Aulenta, W. Hayes, S. Rannard, Eur. Polym. J. 2003, 39, 1741–1771;
- 5cM. J. Cloninger, Curr. Opin. Chem. Biol. 2002, 6, 742–748;
- 5dR. Esfand, D. A. Tomalia, Drug Discovery Today 2001, 6, 427–436;
- 5eA. K. Patri, I. J. Majoros, J. R. Baker, Jr., Curr. Opin. Chem. Biol. 2002, 6, 466–471.
- 6Materials are available in limited supply to other investigators by contacting Professor Simanek.
- 7Inspiration from: C.-H. Wong, X.-S. Ye, Z. Zhang, J. Am. Chem. Soc. 1998, 120, 7137–7138.
- 8
- 8aJ. H. van Boom, P. M. J. Burgers, Tetrahedron Lett. 1976, 17, 4875–4878;
10.1016/S0040-4039(00)78935-0 Google Scholar
- 8bN. Jeker, C. Tamm, Helv. Chim. Acta 1984, 67, 190.
- 9S. Hanessian, P. Lavallee, Can. J. Chem. 1977, 55, 562–565.
- 10J. Connor, A. J. Schroit, Biochemistry 1988, 27, 848–851.
- 11D. A. Westerberg, P. L. Carney, P. E. Rogers, S. J. Kline, D. K. Johnson, J. Med. Chem. 1989, 32, 236–243.
- 12M. B. Steffensen, E. E. Simanek, Org. Lett. 2003, 5, 2359–2361.
- 13
- 13aS. Sinha, P. Ilankumaran, S. Chandrasekaran, Tetrahedron Lett. 1999, 40, 771–774;
- 13bR. G. Bhat, S. Sinha, S. Chandrasekaran, Chem. Commun. 2002, 812–813;
- 13cP. R. Sridhar, S. Chandrasekaran, Org. Lett. 2002, 4, 4731–4733.
- 14H. C. Kolb, M. G. Finn, K. B. Sharpless, Angew. Chem. 2001, 113, 2056–2075;
Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 15Fruit-salad trees are commercially available: http://www.fruitsaladtrees.com/.