The Mechanism of Bu3SnH-Mediated Homolytic Aromatic Substitution
Athelstan L. J. Beckwith Prof.
Research School of Chemistry, Australian National University, Canberra, ACT 0200, Australia, Fax: (+61) 2-6253-0737
Search for more papers by this authorVincent W. Bowry Dr.
Research School of Chemistry, Australian National University, Canberra, ACT 0200, Australia, Fax: (+61) 2-6253-0737
Search for more papers by this authorW. Russell Bowman Prof.
Department of Chemistry, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK, Fax: (+44) 1509-223-925
Search for more papers by this authorEmma Mann Dr.
Department of Chemistry, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK, Fax: (+44) 1509-223-925
Search for more papers by this authorJonathan Parr Dr.
Department of Chemistry, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK, Fax: (+44) 1509-223-925
Search for more papers by this authorJohn M. D. Storey Dr.
Department of Chemistry, Meston Building, University of Aberdeen, Old Aberdeen AB24 3UE, UK, Fax: (+44) 1224-272-921
Search for more papers by this authorAthelstan L. J. Beckwith Prof.
Research School of Chemistry, Australian National University, Canberra, ACT 0200, Australia, Fax: (+61) 2-6253-0737
Search for more papers by this authorVincent W. Bowry Dr.
Research School of Chemistry, Australian National University, Canberra, ACT 0200, Australia, Fax: (+61) 2-6253-0737
Search for more papers by this authorW. Russell Bowman Prof.
Department of Chemistry, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK, Fax: (+44) 1509-223-925
Search for more papers by this authorEmma Mann Dr.
Department of Chemistry, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK, Fax: (+44) 1509-223-925
Search for more papers by this authorJonathan Parr Dr.
Department of Chemistry, Loughborough University, Loughborough, Leicestershire LE11 3TU, UK, Fax: (+44) 1509-223-925
Search for more papers by this authorJohn M. D. Storey Dr.
Department of Chemistry, Meston Building, University of Aberdeen, Old Aberdeen AB24 3UE, UK, Fax: (+44) 1224-272-921
Search for more papers by this authorGraphical Abstract
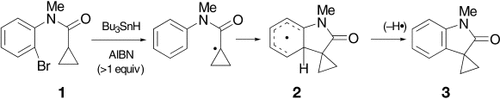
The fate of intermediate π radicals is crucial in Bu3SnH-mediated cyclization by homolytic aromatic substitution, for example, of bromo compound 1 via radical 2 to give oxindole 3 (AIBN=azobisisobutyronitrile). The results indicate that the mechanism requires the abstraction of a hydrogen radical from the intermediate π radical by the radical initiator or a product of initiator breakdown, and also that arene solvents are not always the best solvents for radical reactions.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z52419_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. L. J. Beckwith, J. M. D. Storey, J. Chem. Soc. Chem. Commun. 1995, 977–978.
- 2F. Aldabbagh, W. R. Bowman, E. Mann, A. M. Z. Slawin, Tetrahedron 1999, 55, 8111–8128; W. R. Bowman, H. Heaney, B. M. Jordan, Tetrahedron 1991, 47, 10 119–10 128.
- 3
- 3aW. R. Bowman, E. Mann, J. Parr, J. Chem. Soc. Perkin Trans. 1 2000, 2991–2999;
- 3bS. M. Allin, W. R. S. Barton, W. R. Bowman, T. McInally, Tetrahedron Lett. 2002, 43, 4191–4193.
- 4A detailed list of references is supplied in the Supporting Information. Recent leading references include
- 4aE. Bonfand, L. Forslund, W. B. Motherwell, S. Vázquez, Synlett 2000, 475–478;
C. Escolano, K. Jones, Tetrahedron 2002, 58, 1453–1464;
J. Marco-Contelles, M. Rodríguez-Fernández, Tetrahedron Lett. 2000, 41, 381–384;
A. Nunez, A. G. de Viedma, V. Martínez-Barrasa, C. Burgos, J. Alvarez-Builla, Synlett 2002, 1093–1096; Review:
A. Studer, M. Bossart in Radicals in Organic Synthesis, Vol. 2 (Eds.: ), Wiley-VCH, Weinheim, 2001, pp. 62–80.
10.1002/9783527618293.ch29 Google Scholar
- 5D. C. Harrowven, B. J. Sutton, S. Coulton, Tetrahedron 2002, 58, 3387–3400.
- 6M.-L. Bennasar, T. Roca, R. Griera, J. Bosch, J. Org. Chem. 2001, 66, 7547–7551; M.-L. Bennasar, T. Roca, R. Griera, M. Bassa, J. Bosch, J. Org. Chem. 2002, 67, 6268–6271.
- 7D. Crich, J.-T. Hwang, J. Org. Chem. 1998, 63, 2765–2770.
- 8I. P. Beletskaya, A. S. Sigeev, V. A. Kuzmin, A. S. Tatikolov, L. Hevesi, J. Chem. Soc. Perkin Trans. 2 2000, 107–109.
- 9W. K. Busfield, I. D. Jenkins, P. Van Le, Polym. Bull. 1997, 38, 149–155.
- 10See Table 1, Supporting Information.
- 11D. P. Curran, H. Liu, J. Chem. Soc. Perkin Trans. 1 1994, 1377–1393; D. P. Curran, H. Yu, H. Liu, Tetrahedron 1994, 50, 7343–7366.
- 12P. S. Engel, W.-X. Wu, J. Am. Chem. Soc. 1989, 111, 1830–1835.
- 13The fact that reactions with iodoarenes[5] have been reported to proceed with substoichiometric amounts of AIBN indicates an alternative mechanism involving SET or possibly CI homolysis.