Palladium-Catalyzed Asymmetric [3+3] Cycloaddition of Trimethylenemethane Derivatives with Nitrones†
Ryo Shintani Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorSoyoung Park
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorWei-Liang Duan
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorTamio Hayashi Prof. Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorRyo Shintani Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorSoyoung Park
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorWei-Liang Duan
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorTamio Hayashi Prof. Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo, Kyoto 606-8502, Japan, Fax: (+81) 75-753-3988
Search for more papers by this authorSupport has been provided in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (21 COE on Kyoto University Alliance for Chemistry).
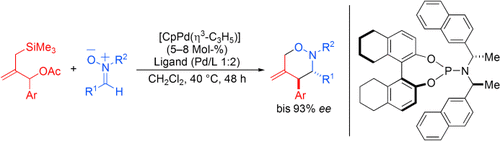
Graphical Abstract
Leichte Cyclisierung: Funktionalisierte 1,2-Oxazine sind durch die palladiumkatalysierte asymmetrische [3+3]-Cycloaddition zwischen Trimethylenmethan-Derivaten und Nitronen zugänglich. Der Einsatz eines modifizierten Phosphoramiditliganden liefert diese Verbindungen mit hoher Stereoselektivität (siehe Schema, Cp=Cyclopentadienyl).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2007/z701529_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. N. Jacobsen, A. Pfaltz, H. Yamamoto, Comprehensive Asymmetric Catalysis I–III, Vol. 3, Springer, Berlin, 1999;
10.1007/978-3-642-58571-5 Google Scholar
- 1bS. Kobayashi, K. A. Jørgensen, Cycloaddition Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2002;
- 1cE. J. Corey, Angew. Chem. 2002, 114, 1724;
10.1002/1521-3757(20020517)114:10<1724::AID-ANGE1724>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1650.10.1002/1521-3773(20020517)41:10<1650::AID-ANIE1650>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 2M. P. Sibi, Z. Ma, C. P. Jasperse, J. Am. Chem. Soc. 2005, 127, 5764.
- 3An example of the non-asymmetric variant was previously reported: R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2006, 128, 6330.
- 4
- 4aB. M. Trost, D. M. T. Chan, J. Am. Chem. Soc. 1979, 101, 6429;
- 4bB. M. Trost, D. M. T. Chan, J. Am. Chem. Soc. 1983, 105, 2315.
- 5For a review, see D. M. T. Chan in Cycloaddition Reactions in Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2002, p. 57.
- 6A. Yamamoto, Y. Ito, T. Hayashi, Tetrahedron Lett. 1989, 30, 375.
- 7B. M. Trost, J. P. Stambuli, S. M. Silverman, U. Schwörer, J. Am. Chem. Soc. 2006, 128, 13328.
- 8For non-asymmetric reactions, see
- 8aR. B. Bambal, R. D. W. Kemmitt, J. Organomet. Chem. 1989, 362, C 18;
- 8bS. J. Hedley, W. J. Moran, A. H. G. P. Prenzel, D. A. Price, J. P. A. Harrity, Synlett 2001, 1596;
- 8cS. J. Hedley, W. J. Moran, D. A. Price, J. P. A. Harrity, J. Org. Chem. 2003, 68, 4286;
- 8dK. M. Goodenough, W. J. Moran, P. Raubo, J. P. A. Harrity, J. Org. Chem. 2005, 70, 207.
- 9The use of [Pd(PPh3)4] as the catalyst gives similar efficiency, but a catalyst generated from [Pd2(dba)3] (dba=trans, trans-dibenzylideneacetone) and PPh3 shows no reactivity.
- 10For examples of metal-catalyzed synthesis of 1,2-oxazines, see
- 10aI. S. Young, M. A. Kerr, Angew. Chem. 2003, 115, 3131; Angew. Chem. Int. Ed. 2003, 42, 3023;
- 10bF. Cardona, A. Goti, Angew. Chem. 2005, 117, 8042;
10.1002/ange.200502640 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7832;
- 10cRef. [2].
- 11B. M. Trost, T. N. Nanninga, T. Satoh, J. Am. Chem. Soc. 1985, 107, 721.
- 12
- 12aT. Hayashi, Acc. Chem. Res. 2000, 33, 354;
- 12bY. Uozumi, A. Tanahashi, S.-Y. Lee, T. Hayashi, J. Org. Chem. 1993, 58, 1945.
- 13H. Takaya, K. Mashima, K. Koyano, M. Yagi, H. Kumobayashi, T. Taketomi, S. Akutagawa, R. Noyori, J. Org. Chem. 1986, 51, 629.
- 14L. A. Arnold, R. Imbos, A. Mandoli, A. H. M. de Vries, R. Zaasz, B. L. Feringa, Tetrahedron 2000, 56, 2865.
- 15B. L. Feringa, M. Pineschi, L. A. Arnold, R. Imbos, A. H. M. de Vries, Angew. Chem. 1997, 109, 2733; Angew. Chem. Int. Ed. Engl. 1997, 36, 2620.
- 16The diastereomer of this compound ((S,R,R)-6 c) has been reported:
- 16aA. Duursma, A. J. Minnaard, B. L. Feringa, Tetrahedron 2002, 58, 5773;
- 16bA. W. van Zijl, L. A. Arnold, A. J. Minnaard, B. L. Feringa, Adv. Synth. Catal. 2004, 346, 413.
- 17A. Alexakis, S. Gille, F. Prian, S. Rosset, K. Ditrich, Tetrahedron Lett. 2004, 45, 1449.
- 18Alkyl-substituted nitrones are not suitable substrates under the present reaction conditions; they give little or no cycloadducts.
- 19CCDC-642825 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc. cam.ac.uk/data request/cif.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.