Solid-State Supramolecular Chirogenesis: High Optical Activity and Gradual Development of Zinc Octaethylporphyrin Aggregates
Victor V. Borovkov Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorTakunori Harada Dr.
Kuroda Chiromorphology Project, ERATO, JST, Park bldg., 4-7-6 Komaba, Meguro-ku, Tokyo 153-0041, Japan, Fax: (+81) 3-5452-0599
Search for more papers by this authorGuy A. Hembury Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorYoshihisa Inoue Prof. Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorReiko Kuroda Prof. Dr.
Kuroda Chiromorphology Project, ERATO, JST, Park bldg., 4-7-6 Komaba, Meguro-ku, Tokyo 153-0041, Japan, Fax: (+81) 3-5452-0599
Search for more papers by this authorVictor V. Borovkov Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorTakunori Harada Dr.
Kuroda Chiromorphology Project, ERATO, JST, Park bldg., 4-7-6 Komaba, Meguro-ku, Tokyo 153-0041, Japan, Fax: (+81) 3-5452-0599
Search for more papers by this authorGuy A. Hembury Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorYoshihisa Inoue Prof. Dr.
Entropy Control Project, ICORP, JST, 4-6-3 Kamishinden, Toyonaka-shi, Osaka 560-0085, Japan, Fax: (+81) 6-6836-1636
Search for more papers by this authorReiko Kuroda Prof. Dr.
Kuroda Chiromorphology Project, ERATO, JST, Park bldg., 4-7-6 Komaba, Meguro-ku, Tokyo 153-0041, Japan, Fax: (+81) 3-5452-0599
Search for more papers by this authorGraphical Abstract
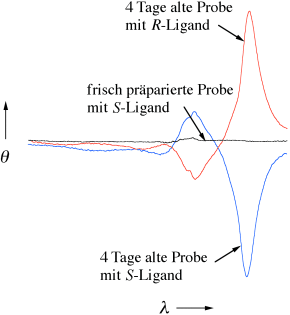
Die langsame Bildung helicaler J-Aggregate erfolgt bei der Wechselwirkung von (achiralem) Zinkoctaethylporphyrin mit enantiomerenreinem 1-Cyclohexylethylamin im festen Zustand. Diese stabilen chiralen Festkörperaggregate zeigen ausgeprägte optische Aktivität, wie die Circulardichroismus-Spektren erkennen lassen (siehe Bild).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z50524_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. M. Ribó, J. Crusats, F. Sagués, J. Claret, R. Rubires, Science 2001, 292, 2063–2066;
- 1bH. Nakashima, J. R. Koe, K. Torimitsu, M. Fujiki, J. Am. Chem. Soc. 2001, 123, 4847–4849;
- 1cJ. M. Fox, T. J. Katz, S. Van Elshocht, T. Verbiest, M. Kauranen, A. Persoons, T. Thongpanchang, T. Kraus, L. Brus, J. Am. Chem. Soc. 1999, 121, 3453–3459;
- 1dR. Purrello, A. Raudino, L. M. Scolaro, A. Loisi, E. Bellacchio, R. Lauceri, J. Phys. Chem. B 2000, 104, 10 900–10 908;
- 1eD. B. Steensgaard, H. Wackerbarth, P. Hildebrandt, A. R. Holzwarth, J. Phys. Chem. B 2000, 104, 10 379–10 386;
- 1fD. Iarossi, A. Mucci, F. Parenti, L. Schenetti, R. Seeber, C. Zanardi, A. Forni, M. Tonelli, Chem. Eur. J. 2001, 7, 676–685;
10.1002/1521-3765(20010202)7:3<676::AID-CHEM676>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 1gM. de Loos, J. van Esch, R. M. Kellogg, B. L. Feringa, Angew. Chem. 2001, 113, 633–636;
10.1002/1521-3757(20010202)113:3<633::AID-ANGE633>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2001, 40, 613–616;10.1002/1521-3773(20010202)40:3<613::AID-ANIE613>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 1hW. Steffen, B. Kohler, M. Altmann, U. Scherf, K. Stitzer, H.-C. zur Loye, U. H. F. Bunz, Chem. Eur. J. 2001, 7, 117–126;
10.1002/1521-3765(20010105)7:1<117::AID-CHEM117>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 1iJ. H. Jung, H. Kobayashi, M. Masuda, T. Shimizu, S. Shinkai, J. Am. Chem. Soc. 2001, 123, 8785–8789;
- 1jL. Brunsveld, E. W. Meijer, R. B. Prince, J. S. Moore, J. Am. Chem. Soc. 2001, 123, 7978–7984;
- 1kM. Wang, G. L. Silva, B. A. Armitage, J. Am. Chem. Soc. 2000, 122, 9977–9986;
- 1lP. Wittung, P. Nielsen, O. Buchart, M. Egholm, B. Norden, Nature 1994, 368, 561–563.
- 2
- 2aV. V. Borovkov, T. Harada, Y. Inoue, R. Kuroda, Angew. Chem. 2002, 114, 1436–1439;
10.1002/1521-3757(20020415)114:8<1436::AID-ANGE1436>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1378–1381;10.1002/1521-3773(20020415)41:8<1378::AID-ANIE1378>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 2bV. V. Borovkov, J. M. Lintuluoto, M. Sugiura, Y. Inoue, R. Kuroda, J. Am. Chem. Soc. 2002, 124, 11 282–11 283;
- 2cV. V. Borovkov, J. M. Lintuluoto, H. Sugeta, M. Fujiki, R. Arakawa, Y. Inoue, J. Am. Chem. Soc. 2002, 124, 2993–3006;
- 2dV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, J. Am. Chem. Soc. 2001, 123, 2979–2989;
- 2eV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, Org. Lett. 2002, 4, 169–171;
- 2fV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, Org. Lett. 2000, 2, 1565–1568;
- 2gV. V. Borovkov, J. M. Lintuluoto, M. Fujiki, Y. Inoue, J. Am. Chem. Soc. 2000, 122, 4403–4407;
- 2hV. V. Borovkov, J. M. Lintuluoto, Y. Inoue, J. Phys. Chem. A 2000, 104, 9213–9219.
- 3The interaction of ZnOEP with other chiral amines in the solid state will be reported elsewhere.
- 4R. Kuroda, Y. Saito, Bull. Chem. Soc. Jpn. 1976, 49, 433–436.
- 5All solid-state samples have been kept in a desiccator under dark conditions.
- 6M. Kasha, H. R. Rawls, M. A. El-Bayoumi, Pure Appl. Chem. 1965, 11, 371–392.
- 7
- 7aR. Rubires, J.-A. Farrera, J. M. Ribó, Chem. Eur. J. 2001, 7, 436–446;
10.1002/1521-3765(20010119)7:2<436::AID-CHEM436>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 7bZ. Bikádi, F. Zsila, J. Deli, G. Mády, M. Simonyi, Enantiomer 2002, 7, 67–76;
- 7cH. von Berlepsch, C. Böttcher, A. Ouart, C. Burger, S. Dähne, S. Kirstein, J. Phys. Chem. B 2000, 104, 5255–5262;
- 7dW. Xu, H. Guo, D. L. Akins, J. Phys. Chem. B 2001, 105, 1543–1546;
- 7eI. Struganova, J. Phys. Chem. A 2000, 104, 9670–9674.
- 8Using a solid-state specialized universal chiroptical spectrophotometer UCS: J-800KCM (R. Kuroda, T. Harada, Y. Shindo, Rev. Sci. Instrum. 2001, 72, 3802–3810) it was shown that optical anisotropies of the solid-state samples are negligibly small (see the Supporting Information). For example, linear dichroism, which is the major source of the artifacts observed in the CD signal of solid-state samples, contributes less than 1 % to the total observed amplitude.
- 9N. Harada, K. Nakanishi, Circular Dichroic Spectroscopy. Exciton Coupling in Organic Stereochemistry, University Science Books, Mill Valley, CA, 1983.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.