A Classical Example of a Disappearing Polymorph and the Shortest Intermolecular H⋅⋅⋅H Separation Ever Found in an Organic Crystal Structure†
Petra Bombicz Dr.
Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, Pusztaszeri út 59–67, PBox 17, 1525 Budapest, Hungary, Fax: (+36) 1-325-7554
Search for more papers by this authorMátyás Czugler Dr.
Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, Pusztaszeri út 59–67, PBox 17, 1525 Budapest, Hungary, Fax: (+36) 1-325-7554
Search for more papers by this authorRoland Tellgren Prof. Dr.
Department of Materials Chemistry, The Ångström Laboratory, Uppsala University, PBox 538, SE-751 21 Uppsala, Sweden
Search for more papers by this authorAlajos Kálmán Prof. Dr.
Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, Pusztaszeri út 59–67, PBox 17, 1525 Budapest, Hungary, Fax: (+36) 1-325-7554
Search for more papers by this authorPetra Bombicz Dr.
Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, Pusztaszeri út 59–67, PBox 17, 1525 Budapest, Hungary, Fax: (+36) 1-325-7554
Search for more papers by this authorMátyás Czugler Dr.
Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, Pusztaszeri út 59–67, PBox 17, 1525 Budapest, Hungary, Fax: (+36) 1-325-7554
Search for more papers by this authorRoland Tellgren Prof. Dr.
Department of Materials Chemistry, The Ångström Laboratory, Uppsala University, PBox 538, SE-751 21 Uppsala, Sweden
Search for more papers by this authorAlajos Kálmán Prof. Dr.
Institute of Chemistry, Chemical Research Center, Hungarian Academy of Sciences, Pusztaszeri út 59–67, PBox 17, 1525 Budapest, Hungary, Fax: (+36) 1-325-7554
Search for more papers by this authorThe authors express their gratitude to Prof. Jack Dunitz for invaluable discussions. The authors acknowledge Dr. József Kovács, Mr. Csaba Kertész, and Dr. János Madarász (Budapest), Mr. Håkan Rundlöf and Dr. Valéry Baron (Studsvik). P.B. is indebted to the Royal Swedish Academy of Sciences for the support of her stay at the Studsvik Neutron Research Laboratory, Uppsala University.
Graphical Abstract
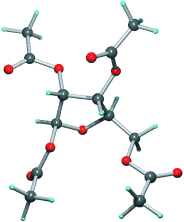
Taucht nur alle Jubeljahre auf: Neutronen- und Röntgenbeugungsuntersuchungen bei tiefen Temperaturen ergaben ein empfindliches Gleichgewicht zwischen anziehenden und abstoßenden intermolekularen Kräften in den beiden kristallinen Formen von 1,2,3,5-Tetra-O-acetyl-β-D-ribofuranose (zu sehen ist die weniger stabile Form; O: rot, H: blau, C: grau). Sehr kleine intermolekulare H⋅⋅⋅H-Abstände, die nur in der weniger stabilen Kristallform vorliegen, sind für deren Verschwinden maßgeblich.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z19504_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. D. Dunitz, J. Bernstein, Acc. Chem. Res. 1995, 28, 193–200.
- 2J. Webb, B. Anderson, J. Chem. Educ. 1978, 55, 644; G. D. Woodard, W.C McCrone, J. Appl. Crystallogr. 1975, 8, 342.
- 3J. Bernstein, J. O. Henck, Cryst. Eng. 1998, 1, 119–128.
- 4J. P. Glusker, M. Lewis, M. Rossi, Crystal Structure Analysis for Chemists and Biologists, VCH, New York, 1994; J. Dunitz, Acta Crystallogr. Sect. A 1999, 55, 2.
- 5V. W. Jacewicz, J. H. C. Nayler, J. Appl. Crystallogr. 1979, 12, 396–397.
- 6“Polymorphism in Molecular Crystals”: J. Bernstein in IUCr Monographs on Crystallography 14, Clarendon, Oxford, 2002.
- 7“Organic Solid State Chemistry”: G. R. Desiraju in Studies in Organic Chemistry 32, Elsevier, Amsterdam, 1987, p. 489.
- 8G. A. Howard, B. Lythgoe, A. R. Todd, J. Chem. Soc. 1947, 1052–1054.
- 9H. Bredereck, E. Hoepfner, Chem. Ber. 1948, 81, 51–52.
- 10H. Zinner, Chem. Ber. 1950, 83, 153–156.
- 11R. D. Guthrie, S. C. Smith, Chem. Ind. 1968, 547–548.
- 12J. Davoll, G. B. Brown, D. V. Visser, Nature 1952, 170, 64–65.
- 13K. R. Farrar, Nature 1952, 170, 896.
- 14A. L. Patterson, B. P. Groshens, Nature 1954, 173, 398.
- 15V. J. James, J. D. Stevens, Cryst. Struct. Commun. 1973, 2, 609–612.
- 16B. J. Poppleton, Acta Crystallogr. Sect. B. 1976, 32, 2702–2705.
- 17M. Czugler, A. Kálmán, J. Kovács, I. Pintér, Acta Crystallogr. Sect. B 1981, 37, 172–177.
- 18“Crystal Engineering, The Design of Organic Solids”: G. R. Desiraju in Material Science Monographs, Vol. 5, Elsevier, Amsterdam, 1989.
- 19T. L. Threlfall, Analyst 1995, 120, 2435–2460; A. Nangia, G. R. Desiraju, Top. Curr. Chem. 1998, 198, 57–96.
- 20G. R. Desiraju, Nature 2001, 412, 397–400.
- 21S. R. Chemburkar, J. Bauer, K. Deming, H. Spiwek, K. Patel, J. Morris, R. Henry, S. Spanton, W. Dziki, W. Porter, J. Quick, P. Bauer, J. Donaubauer, B. A. Narayanan, M. Soldani, D. Riley, K. McFarland, Org. Process Res. Dev. 2000, 4, 413–417.
- 22“Polymorphism”: W. C. McCrone in Physics and Chemistry of the Organic Solid State, Vol. II (Ed ), Interscience, New York, 1965, pp. 726–767.
- 23M. R. Caira, Top. Curr. Chem. 1998, 198, 163–208.
- 24L. Addadi, Z. Berkovitch-Yellin, I. Weissbuch, J. van Mil, L. Shimon, M. Lahav, L. Leiserowitz, Angew. Chem. 1985, 97, 476–495; Angew. Chem. Int. Ed. Engl. 1985, 24, 439–528; Z. Berkovitch-Yellin, J. van Mil, L. Addadi, M. Idelson, M. Lahav, L. Leiserowitz, J. Am. Chem. Soc. 1985, 107, 3111–3122; E. Staab, L. Addadi, L. Leiserowitz, M. Lahav, Adv. Mater. 1990, 2, 40–43.
- 25R. J. Davey, L. A. Polywka, S. J. Maginn, The Control of Morhology by Additives: Molecular Recognition, Kinetics and Technology, Advances in Industrial Crystallization (Ed ), Butterworth-Heinemann, Oxford, 1991, pp. 150–165.
- 26A. Bondi, J. Phys. Chem. 1964, 68, 441–451.
- 27R. S. Rowland, R. Taylor, J. Phys. Chem. 1996, 100, 7384–7391.
- 28D. Braga, G. R. Desiraju, J. S. Miller, A. G. Orpen, S. L. Price, CrystEngComm 2002, 4, 500–509.
- 29M. J. Calhorda, P. J. Costa, CrystEngComm 2002, 4, 368–372.
- 30X-ray analyses of both crystal forms at 110 K, MoKα radiation λ=0.71073 Å, scan mode ω-θ; Lorentz and polarization correction, structure solution by direct methods with the program SHELXS97[31a] least-squares structure refinement on F2 by SHELXL[31b] form A: crystal dimensions: 0.8×0.5×0.5 mm, monoclinic, P21, a=12.515(3), b=5.464(1), c=10.863(2) Å, β=96.86(3)°, V=737.5(3) Å3, Z=2, ρcalcd=1.433 g cm−3, 6309 collected reflections, 6098 independent reflections, 5452 reflections I>2σ, θmax=55.09°, parameters 271, μ=0.123, no absorption correction, R=0.046, wR2=0.136, residual electron density 0.436 and −0.404 e Å−3; form B: crystal dimensions: 0.7×0.5×0.35 mm, orthorhombic, P212121, a=7.360(3), b=13.596(4), c=15.183(4) Å, V=1519.3(9) Å3, Z=4, ρcalcd=1.391 g cm−3, 12 953 collected reflections, 11 769 independent reflections, 7791 reflections I>2σ, θmax=55.09°, parameters 271, μ=0.119, semi-empirical absorption correction, Tmin=0.963, Tmax=0.998, R=0.045, wR2=0.122, residual electron density 0.357 and −0.363 e Å−3. All hydrogen atom positions were located in the difference electron density maps, none of them appeared disordered. CCDC 174273, CCDC 174274, CCDC 174275, and CCDC 174276 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223–336–033; or [email protected]).
- 31aG. M. Sheldrick, SHELXS-97, Program for Crystal Structure Solution (Release 97-2), University of Göttingen, Göttingen (Germany), 1997;
- 31bG. M. Sheldrick, SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen (Germany), 1997.
- 32
- 32aNeutron diffraction measurements at 25 K, scan mode ω-θ; Lorentz and polarization correction, least-squares structure refinement on F by DUPALS,[32b] form A: λ=1.215 Å, crystal dimensions: 2×4×4 mm, a=12.592(4), b=5.463(2), c=10.867(4) Å, β=96.355(1)°, V=742.9(7) Å3, Z=2, ρcalcd=1.423 g cm−3, 1666 collected reflection, 1512 independent reflections, 1450 reflections I>3σ, θmax=60.00°, parameters 251, μ=0.2055, analytical absorption correction, Tmin=0.3587, Tmax=0.7013, R=0.031; form B: λ=1.207 Å, crystal dimensions: 1×2.6×3 mm, a=7.291(4), b=13.577(4), c=15.117(9) Å, V=1496.4(7) Å3, Z=4, ρcalcd=1.412 g cm−3 2237 collected reflection, 2033 independent reflections, 1430 reflections I>2σ, θmax=51.9°, parameters 252, μ=0.233, analytical absorption correction, Tmin=0.6262, Tmax=0.8032, R=0.059;
- 32bT. Gustafsson, DUPALS, A Full-matrix Least-squares Refinement Program, Uppsala University, 1993.
- 33J. D. Dunitz, A. Gavezotti, Acc. Chem. Res. 1999, 32, 677–684.
- 34
- 34aF. H. Allen, W. D. S. Motherwell, Acta Crystallogr. Sect. B 2002, 58, 407–422;
- 34bCSD October 2000 release. 80 structures with 130 close contacts were retrieved among the 574 neutron data found with the search criteria: 3D coordinates published, no errors, not disordered, no H3O+⋅⋅⋅H2O ionic contacts and R factor is less than 0.075. The shortest H⋅⋅⋅H distance found in the Database is in nicotinamide (NICOAM03)[35] with 1.979(4) Å.
- 35Y. Miwa, T. Mizuno, K. Tsuchida, T. Taga, Y. Iwata, Acta Crystallogr. Sect. B 1999, 55, 78–84.
- 36R. L. Rowley, T. Pakkanen, J. Chem. Phys. 1999, 110, 3368–3377.
- 37A. Gavezzotti, G. Filippini, J. Am. Chem. Soc. 1995, 117, 12 299–12 305.
- 38A. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 2002.
- 39T. Steiner, W. Hinrichs, W. Saenger, Acta Crystallogr. Sect. B 1993, 49, 708–718.
- 40J. D. Dunitz, G. Filippini, A. Gavezotti, Tetrahedron 2000, 56, 6595–6601.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.