Acetylene Hydratase
Abstract
The tungsten-iron-sulfur enzyme acetylene hydratase is a rather unique enzyme within the class of tungsten/molybdenum enzymes in the sense that it catalyzes a nonredox reaction, the addition of one molecule of water to the CC bond of acetylene to form acetaldehyde. Its crystal structure (1.26 Å) reveals a close to octahedral, or trigonal antiprismatic tungsten center, which binds a water molecule that gets activated by an adjacent aspartate residue such that it can attack an acetylene molecule bound in a distinct, hydrophobic pocket. This requires a strong shift of pKa of the aspartate, caused by a nearby low-potential [4Fe–4S] cluster. To gain access to this novel W-Asp-active site, the protein evolved a new substrate channel distant from where it is found in other molybdenum and tungsten enzymes.
3D Structure
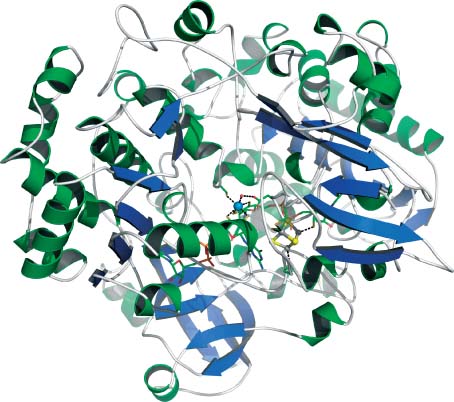
Three-dimensional structure of AH from Pelobacter acetylenicus.4 Tungsten is shown as a blue sphere; iron and sulfide atoms of the [4Fe–4S] cluster are shown as gray and yellow spheres. The two MGD ligands of tungsten are shown as sticks (sulfur = yellow). Produced with the program PyMOL.20 PDB code: 2E7Z.