Poly(p-phenylenephosphaalkene): A π-Conjugated Macromolecule Containing PC Bonds in the Main Chain†
Vincent A. Wright
Department of Chemistry University of British Columbia 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada) Fax: (+1) 604-822-2847
Search for more papers by this authorDerek P. Gates Prof.
Department of Chemistry University of British Columbia 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada) Fax: (+1) 604-822-2847
Search for more papers by this authorVincent A. Wright
Department of Chemistry University of British Columbia 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada) Fax: (+1) 604-822-2847
Search for more papers by this authorDerek P. Gates Prof.
Department of Chemistry University of British Columbia 2036 Main Mall, Vancouver, BC, V6T 1Z1 (Canada) Fax: (+1) 604-822-2847
Search for more papers by this authorWe thank the Natural Sciences and Engineering Research Council (NSERC) of Canada and the University of British Columbia for support of this work, and Prof. M. Wolf for the use of UV/Vis and IR equipment.
Graphical Abstract
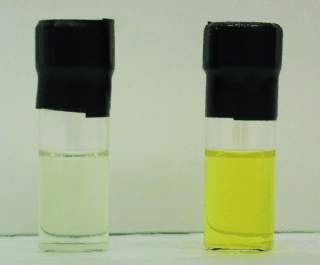
An unprecedented yellow polymer with low-coordinate phosphorus atoms in the backbone has been prepared. The material is soluble in polar organic solvents, and moderate molecular weights (Mn=2900–10 500 g mol−1) were estimated from 31P NMR spectroscopic end-group analysis. The possible π-conjugation was investigated by UV/Vis spectroscopy, which revealed a red shift in λmax for the polymer when compared with colorless molecular-model systems (see picture; left: model system, right: new polymer, in THF).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z18848_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Selected early breakthroughs in the synthesis of stable compounds containing PC, PP, SiC, SiSi, PC bonds:
- 1a G. Becker, Z. Anorg. Allg. Chem. 1976, 423, 242;
- 1b T. C. Klebach, R. Lourens, F. Bickelhaupt, J. Am. Chem. Soc. 1978, 100, 4886;
- 1c M. Yoshifuji, I. Shima, N. Inamoto, K. Hirotsu, T. Higuchi, J. Am. Chem. Soc. 1981, 103, 4587;
- 1d A. G. Brook, F. Abdesaken, B. Gutekunst, G. Gutekunst, R. K. Kallury, J. Chem. Soc. Chem. Commun. 1981, 191;
- 1e R. West, M. J. Fink, J. Michl, Science 1981, 214, 1343;
- 1f G. Becker, G. Gresser, W. Uhl, Z. Naturforsch. B 1981, 36, 16.
- 2 For reviews, see: P. P. Power, Chem. Rev. 1999, 99, 3463; R. Okazaki, N. Tokitoh, Acc. Chem. Res. 2000, 33, 625; M. Yoshifuji, J. Chem. Soc. Dalton Trans. 1998, 3343; L. Weber, Chem. Ber. 1996, 129, 367; N. C. Norman, Polyhedron 1993, 12, 2431; E. Niecke, D. Gudat, Angew. Chem. 1991, 103, 251; Angew. Chem. Int. Ed. Engl. 1991, 30, 217; M. Regitz, Chem. Rev. 1990, 90, 191; R. West, Angew. Chem. 1987, 99, 1231; Angew. Chem. Int. Ed. Engl. 1987, 26, 1201; A. H. Cowley, Polyhedron 1984, 3, 389.
- 3 For reviews, see:
- 3a Handbook of Conducting Polymers, 2 ed. (Eds.: T. A. Skotheim, R. L. Elsenbaumer, J. R. Reynolds), Dekker, New York, 1998;
- 3b
A. Kraft, A. C. Grimsdale, A. B. Holmes, Angew. Chem. 1998, 110, 416;
10.1002/(SICI)1521-3757(19980216)110:4<416::AID-ANGE416>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1998, 37, 402;10.1002/(SICI)1521-3773(19980302)37:4<402::AID-ANIE402>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 3c W. J. Feast, J. Tsibouklis, K. L. Pouwer, L. Groenendaal, E. W. Meijer, Polymer 1996, 37, 5017;
- 3d U. H. F. Bunz, Chem. Rev. 2000, 100, 1605;
- 3e D. T. McQuade, A. E. Pullen, T. M. Swager, Chem. Rev. 2000, 100, 2537.
- 4 The spontaneous polymerization of PhCP has been reported. NMR spectroscopic analysis suggests that the polymer is mainly composed of saturated trivalent phosphane moieties with PC units being a minor component. D. A. Loy, G. M. Jamison, M. D. McClain, T. M. Alam, J. Polym. Sci. Part A 1999, 37, 129.
- 5
The intriguing polymeric metal, (SN)x, is a superconductor at 0.26 K, however the electronic structure of this solid-state inorganic material is still under investigation. For a recent review, see: A. J. Banister, I. B. Gorrell, Adv. Mater. 1998, 10, 1415.
10.1002/(SICI)1521-4095(199812)10:17<1415::AID-ADMA1415>3.0.CO;2-L CAS Web of Science® Google Scholar
- 6
- 6a
I. Manners, Angew. Chem. 1996, 108, 1712;
10.1002/ange.19961081504 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1602;
- 6b H. R. Allcock, Adv. Mater. 1994, 6, 106;
- 6c J. E. Mark, H. R. Allcock, R. West, Inorganic Polymers, Prentice Hall, New Jersey, 1992.
- 7
For recent examples of conjugated polymers containing inorganic elements, see: M. Altmann, U. H. F. Bunz, Angew. Chem. 1995, 107, 603;
10.1002/ange.19951070509 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 569; H. A. Brison, T. P. Pollagi, T. C. Stoner, S. J. Geib, M. D. Hopkins, Chem. Commun. 1997, 1263; N. Matsumi, K. Naka, Y. Chujo, J. Am. Chem. Soc. 1998, 120, 5112; H. Sohn, R. R. Huddleston, D. R. Powell, R. West, K. Oka, X. Yonghua, J. Am. Chem. Soc. 1999, 121, 2935; B. L. Lucht, M. A. Buretea, T. D. Tilley, Organometallics 2000, 19, 3469; S. Yamaguchi, T. Goto, K. Tamao, Angew. Chem. 2000, 112, 1761;10.1002/(SICI)1521-3757(20000502)112:9<1761::AID-ANGE1761>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1695;10.1002/(SICI)1521-3773(20000502)39:9<1695::AID-ANIE1695>3.0.CO;2-O CAS PubMed Web of Science® Google ScholarB. L. Lucht, St. Onge, Chem. Commun. 2000, 2097.
- 8 J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns, A. B. Holmes, Nature 1990, 347, 539.
- 9 See for example: K. B. Dillon, F. Mathey, J. F. Nixon, Phosphorus: The Carbon Copy, Wiley, New York, 1998; R. Appel in Multiple Bonds and Low Coordination in Phosphorus Chemistry ( ), Thieme, Stuttgart, 1990; J. F. Nixon, Chem. Rev. 1988, 88, 1327; F. Mathey, Acc. Chem. Res. 1992, 25, 90; L. Weber, Eur. J. Inorg. Chem. 2000, 2425.
- 10 The poly(azomethines), CN analogues of PPV, have been known since the 1920s (R. Adams, R. E. Bullock, W. C. Wilson, J. Am. Chem. Soc. 1923, 45, 521) and soluble derivatives exhibiting photoluminescence, liquid crystallinity, high thermal stability, and high tensile strength are known. See for example: P. W. Morgan, S. L. Kwolek, T. C. Pletcher, Macromolecules 1987, 20, 729; T. Matsumoto, F. Yamada, T. Kurosaki, Macromolecules 1997, 30, 3547; O. Thomas, O. Inganäs, M. R. Andersson, Macromolecules 1998, 31, 2676.
- 11 See, for example:
- 11a G. Becker, Z. Anorg. Allg. Chem. 1977, 430, 66;
- 11b G. Becker, O. Mundt, Z. Anorg. Allg. Chem. 1978, 443, 53;
- 11c G. Becker, W. Becker, G. Uhl, Z. Anorg. Allg. Chem. 1984. 518, 21;
- 11d R. Pietschnig, E. Niecke, M. Nieger, K. Airola, J. Organomet. Chem. 1997, 529, 127;
- 11e A. Grünhagen, U. Pieper, T. Kottke, H. W. Roesky, Z. Anorg. Allg. Chem. 1994, 620, 716;
- 11f A. Mack, E. Pierron, T. Allspach, U. Bergsträßer, M. Regitz, Synthesis 1998, 1305.
- 12 See, for example:
- 12a A. Jouaiti, M. Geoffroy, G. Terron, G. Bernardinelli, J. Chem. Soc. Chem. Commun. 1992, 155;
- 12b F. Knoch, R. Appel, H. Wenzel, Z. Krystallogr. 1995, 210, 224;
- 12c A. Jouaiti, M. Geoffroy, G. Terron, G. Bernardinelli, J. Am. Chem. Soc. 1995, 117, 2251;
- 12d H. Kawanami, K. Toyota, M. Yoshifuji, Chem. Lett. 1996, 533;
- 12e S. Shah, T. Concolino, A. L. Rheingold, J. D. Protasiewicz, Inorg. Chem. 2000, 39, 3860.
- 13 E. M. Evleth, L. D. Freeman, R. I. Wagner, J. Org. Chem. 1962, 27, 2192.
- 14 The phosphane (5) was mentioned previously, however, detailed synthetic procedures were not described. R. Appel, P. Fölling, B. Josten, W. Schuhn, H. V. Wenzel, F. Knoch, Z. Anorg. Allg. Chem. 1988, 556, 7. Our synthetic procedure and spectroscopic data are provided in the Supporting Information.
- 15 Heating the polymerization mixture for 48 h at 85° C resulted in an insoluble yellow gel which swelled reversibly in THF. Analysis of the swollen gel by 31P NMR spectroscopy showed broad resonances similar to those for the soluble polymer 3. Presumably, this material is partially cross-linked or high molecular weight 3.
- 16 Samples of 3 exhibit no change in their NMR spectra after several months of storage in THF solution under an inert atmosphere. Upon exposure to moisture, solutions of 3 rapidly undergo partial hydrolysis, and signals arising from -PH2 and -PHSiMe3 end groups were observed by using 31P NMR spectroscopy. Excess water results in partial hydrolysis of the O-SiMe3 side groups giving an enol, which tautomerizes to acylphosphane (δ=−16 ppm; 1JPH=232 Hz).
- 17 The molecular weights of 3 were estimated by integration of the P(SiMe3)2 and PC signals in the 31P NMR spectrum (relaxation delays of between 2 and 30 s resulted in identical ratios). A statistical (50:50) mixture of C(O)Cl and P(SiMe3)2 end groups was assumed; consistent with elemental analysis and the trace of C(O)Cl (δ=170 ppm) detected in the baseline of the 13C NMR spectrum. We speculate that the small resonance at 50 ppm in the 31P NMR spectrum of 3 is caused by minor cross-linking of the polymer chains. To date, the sensitivity of 3 towards oxygen and moisture has precluded GPC analysis. Thus far, MALDI-TOF MS has not been successful, perhaps because of the reactivity of 3 with hydroxy-containing matrices.
- 18 H. Kawanami, K. Toyota, M. Yoshifuji, J. Organomet. Chem. 1997, 535, 1.
- 19
Incorporation of 2,3,5,6-tetramethyl-1,4-phenylene units into PPV leads to a blue shift of 20–30 nm in the absorbance spectrum. See S. Chung, D. W. Lee, D. Oh, C. E. Lee, J. Jin, Acta Polym. 1999, 50, 298.
10.1002/(SICI)1521-4044(19990801)50:8<298::AID-APOL298>3.0.CO;2-Z CAS Web of Science® Google Scholar
- 20 W. W. Simmons, The Sadtler Handbook of Ultraviolet Spectra, Sadtler Research Laboratories, Philadelphia, 1979.
- 21 For a discussion of the NMR spectra of phosphaalkenes see E. Fluck in Topics in Phosphorus Chemistry, Vol. 10, Wiley, New York, 1980, p. 193, and references therein.



41:13<>1.0.co;2-h.cover.gif)
