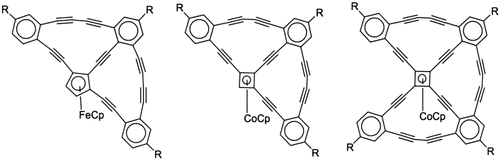
Synthesis and Structural Characterization of Organometallic Cyclynes: Novel Nanoscale, Carbon-Rich Topologies†
Matthew Laskoski
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorWinfried Steffen
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorJason G. M. Morton
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorMark D. Smith Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorUwe H. F. Bunz Prof. Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorMatthew Laskoski
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorWinfried Steffen
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorJason G. M. Morton
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorMark D. Smith Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorUwe H. F. Bunz Prof. Dr.
Department of Chemistry and Biochemistry The University of South Carolina Columbia, SC 29208 (USA) Fax: (+1) 803-777-929-0267
Search for more papers by this authorWe thank the National Science Foundation for generous support (CAREER award; CHE 99-81765).
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z18746_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 M. D. Watson, A. Fechtenkötter, K. Müllen, Chem. Rev. 2001, 101, 1267; A. J. Berresheim, M. Müller, K. Müllen, Chem. Rev. 1999, 99, 1747; S. Ito, P. T. Herwig, T. Böhme, J. P. Rabe, W. Rettig, K. Müllen, J. Am. Chem. Soc. 2000, 122, 7698; F. Dötz, J. D. Brand, S. Ito, L. Gherghel, K. Müllen, J. Am. Chem. Soc. 2000, 122, 7707; T. Weil, U. M. Wiesler, A. Herrmann, R. Bauer, J. Hofkens, F. C. De Schryver, K. Müllen, J. Am. Chem. Soc. 2001, 123, 8101.
- 2 R. Diercks, J. C. Armstrong, R. Boese, K. P. C. Vollhardt, Angew. Chem. 1986, 98, 270; Angew. Chem. Int. Ed. Engl. 1986, 25, 268; P. I. Dosa, C. Erben, V. S. Iyer, K. P. C. Vollhardt, I. M. Wasser, J. Am. Chem. Soc. 1999, 121, 10 430; R. Boese, A. J. Matzger, K. P. C. Vollhardt, J. Am. Chem. Soc. 1997, 119, 2052.
- 3 R. Dembinski, T. Bartik, B. Bartik, M. Jaeger, J. A. Gladysz, J. Am. Chem. Soc. 2000, 122, 810.
- 4 C. Kosinski, A. Hirsch, F. W. Heinemann, F. Hampel, Eur. J. Org. Chem. 2001, 3879; B. Leibrock, O. Vostrowsky, A. Hirsch, Eur. J. Org. Chem. 2001, 4401; G. Schermann, T. Grösser, F. Hampel, A. Hirsch, Chem. Eur. J. 1997, 3, 1105.
- 5
Y. Rubin, T. C. Parker, S. J. Pastor, S. Jalisatgi, C. Boulle, C. L. Wilkins, Angew. Chem. 1998, 110, 1353;
10.1002/(SICI)1521-3757(19980504)110:9<1353::AID-ANGE1353>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 1226;10.1002/(SICI)1521-3773(19980518)37:9<1226::AID-ANIE1226>3.0.CO;2-H CAS PubMed Web of Science® Google ScholarN. Jux, K. Holczer, Y. Rubin, Angew. Chem. 1996, 108, 2031;10.1002/ange.19961081733 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1986.
- 6
For an excellent review, see M. M. Haley, J. J. Pak, S. C. Brand, Top. Curr. Chem. 1999, 201, 82;
W. B. Wan, S. C. Brand, J. J. Pak, M. M. Haley, Chem. Eur. J. 2000, 6, 2044;
10.1002/1521-3765(20000602)6:11<2044::AID-CHEM2044>3.0.CO;2-Y CAS PubMed Web of Science® Google ScholarM. M. Haley, S. C. Brand, J. J. Pak, Angew. Chem. 1997, 109, 864;10.1002/ange.19971090808 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 836; J. J. Pak, T. J. R. Weakley, M. M. Haley, Organometallics 1997, 16, 4505.
- 7 F. Diederich, Nature 1994, 369, 199; F. Diederich, Y. Rubin, Angew. Chem. 1992, 104, 1123; Angew. Chem. Int. Ed. Engl. 1992, 31, 1101; J. D. Tovar, N. Jux, T. Jarrosson, S. I. Khan, Y. Rubin, J. Org. Chem. 1997, 62, 3432.
- 8
G. J. Palmer, S. R. Parkin, J. E. Anthony, Angew. Chem. 2001, 113, 2577;
10.1002/1521-3757(20010702)113:13<2577::AID-ANGE2577>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2509.10.1002/1521-3773(20010702)40:13<2509::AID-ANIE2509>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 9 Y. Tobe, K. Kubota, K. Naemura, J. Org. Chem. 1997, 62, 3430.
- 10 For an example of an organometallic cyclyne, see J. D. Bradshaw, L. Guo, C. A. Tessier, W. J. Youngs, Organometallics 1996, 15, 2582.
- 11
G. Scholz, R. Gleiter, F. Rominger, Angew. Chem. 2001, 113, 2559;
Angew. Chem. Int. Ed. 2001, 40, 2477.
10.1002/1521-3773(20010702)40:13<2477::AID-ANIE2477>3.0.CO;2-J PubMed Web of Science® Google Scholar
- 12 U. H. F. Bunz, Top. Curr. Chem. 1999, 201, 131; U. H. F. Bunz, Y. Rubin, Y. Tobe, Chem. Soc. Rev. 1999, 107.
- 13
M. Laskoski, G. Roidl, M. D. Smith, F Bunz, Angew. Chem. 2001, 113, 1508;
10.1002/1521-3757(20010417)113:8<1508::AID-ANGE1508>3.0.CO;2-7 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1460; M. Laskoski, M. D. Smith, J. G. M. Morton, U. H. F. Bunz, J. Org. Chem. 2001, 66, 5174; U. H. F. Bunz, G. Roidl, M. Altmann, V. Enkelmann, K. D. Shimizu, J. Am. Chem. Soc. 1999, 121, 10 719.
- 14
- 14a M. Laskoski, J. G. M. Morton, M. D. Smith, U. H. F. Bunz, Chem. Commun. 2001, 2590;
- 14b for the active catalyst [Pd{P(o-tolyl)3}2], see F. Paul, J. Patt, J. F. Hartwig, Organometallics 1995, 14, 3030.
- 15
- 15a S. Ohira, Synth. Lett. 1989, 19, 561; S. Müller, B. Liepold, G. J. Roth, H. J. Bestmann, Synlett 1996, 521; D. F. Taber, Y. Wang, J. Am. Chem. Soc. 1997, 119, 22;
- 15b for the diazophosphonate, see D. Seyferth, R. S. Marmor, P. Hilbert, J. Org. Chem. 1971, 36, 1379; D. G. Brown, E. J. Velthuisen, J. R. Commerford, R. G. Brisbois, T. R. Hoye, J. Org. Chem. 1996, 61, 2540;
- 15c
the most efficient alkyne–alkyne coupling reaction we have utilized so far has been developed by: F. Vögtle, R. Berscheid, Synthesis 1992, 58;
P. Siemsen, R. C. Livingston, F. Diederich, Angew. Chem. 2000, 112, 2740;
10.1002/1521-3757(20000804)112:15<2740::AID-ANGE2740>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000 39, 2633.
- 16 M. M. Haley, personal communication on related transformations.
- 17 For details of X-ray crystallography on 7, 12, and 17 see the Supporting Information. Crystal data for 7: C51H37Co, Mr=708.74, triclinic, P-1, a=10.9507(8), b=12.1458(8), c=15.4401(11) Å, α=86.981(1), β=79.657(1), γ=65.458(1)°, V=1837.2(2) Å3, Z=2, ρcalcd=1.281 g cm−3. 2θmax=50.2°; 15 359 reflections collected, 6532 independent, 5052 with I>2σ(I). No absorption correction (μ=0.50 mm−1). R1, wR2 (I>2σ(I))=0.0416, 0.814, respectively.
- 18 Crystal data for 12: C51H36Fe⋅0.25 CH2Cl2, Mr=725.88, hexagonal, P63, a=27.174(2), c=9.1170(7) Å, V=5830.4(7) Å3, Z=6, ρcalcd=1.240 g cm−3. 2θmax=48.2°; 22 326 reflections collected, 5788 independent, 4316 with I>2σ(I). No absorption correction applied (μ=0.46 mm−1). R1, wR2 (I> 2σ(I))=0.0623, 0.1231, respectively; GOF=1.013. 509 parameters refined, 4 restraints (disordered CH2Cl2 solvent).
- 19 Crystal data for 17: C69H59Co, Mr=939.03, monoclinic, P21/n, a=10.568(1), b=35.490(4), c=15.132(2) Å, β=102.215(3)°, V=5547.0(11) Å3, Z=4, ρcalcd=1.124 g cm−3. 2θmax=45.1°; 21 290 reflections collected, 7278 independent, 3104 with I>2σ(I). No absorption correction (μ=0.35 mm−1). R1, wR2 (I>2σ(I))=0.1098, 0.2544, repectively; GOF=1.008. 605 parameters refined. Molecular disorder corresponding to a 90° rotation around the Co⋅⋅⋅Cpcentroid vector is present, but could not be modeled as a result of the small fraction (<10 %).
- 20 U. H. F. Bunz, V. Enkelmann, Organometallics 1994, 13, 3823.
- 21 W. Jentzen, M. C. Simpson, J. D. Hobbs, X. Song, T. Ema, N. Y. Nelson, C. J. Medforth, K. M. Smith, M. Veyrat, M. Mazzanti, R. Rammaseul, J. C. Marchon, T. Takeuchi, W. A. Goddard, J. A. Shellnutt, J. Am. Chem. Soc. 1995, 117, 11 085.
- 22 For an example of a hexagonal fenestrane geometry, see W. B. Wan, M. M. Haley, J. Org. Chem. 2001, 66, 3893.
- 23
For examples of planar carbon and bona fide fenestrane geometries in organic and organometallic chemistry, see D. Röttgers, G. Erker, R. Fröhlich, M. Grehl, S. J. Silvero, I. Hyla-Kryspin, R. Gleiter, J. Am. Chem. Soc. 1995, 117, 10 503;
10.1021/ja00147a011 Google ScholarM. Thommen, R. Keese, Synlett 1997, 231; D. Kuck, A. Schuster, R. A. Krause J. Org. Chem. 1991, 56, 3472; S. Grimme, J. Am. Chem. Soc. 1996, 118, 1529; D. Kuck, Chem. Ber. 1994, 127, 409.



41:13<>1.0.co;2-h.cover.gif)
