Bifunctional Cp∩N Complexes—Unusual Structural Features and Electronic Coupling in Highly Preorganized Bimetallic Systems†
Jens C. Röder Dr.
Anorganisch-Chemisches Institut Universität Heidelberg Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorFranc Meyer Prof. Dr.
Institut für Anorganische Chemie Georg-August-Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-39-3063
Search for more papers by this authorElisabeth Kaifer Dr.
Anorganisch-Chemisches Institut Universität Heidelberg Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorJens C. Röder Dr.
Anorganisch-Chemisches Institut Universität Heidelberg Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorFranc Meyer Prof. Dr.
Institut für Anorganische Chemie Georg-August-Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-39-3063
Search for more papers by this authorElisabeth Kaifer Dr.
Anorganisch-Chemisches Institut Universität Heidelberg Im Neuenheimer Feld 270, 69120 Heidelberg (Germany)
Search for more papers by this authorGenerous support by Prof. Dr. G. Huttner is gratefully acknowledged. We thank the DFG (SFB 247; Graduiertenkolleg-Stipendium to J.C.R.) and the Fonds der Chemischen Industrie for funding.
Graphical Abstract
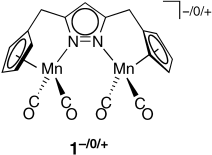
Fast electron transfer between the Mn centers in the mixed-valent complex 1, a dinuclear analogue of complexes with Cp′-N-ligands (Cp=C5H5), supports the occurrence of cooperative effects in such highly preorganized bimetallic systems. An unsual η1:η1:η5 coordination of the pyrazolate group is observed for K+ 1− in the solid state.
References
- 1
- 1a J. Okuda, Comments Inorg. Chem. 1994, 16, 185–205;
- 1b P. Jutzi, U. Siemeling, J. Organomet. Chem. 1995, 500, 175–185.
- 2 A. L. McKnight, R. M. Waymouth, Chem. Rev. 1998, 98, 2587–2598.
- 3 J. C. Röder, F. Meyer, H. Pritzkow, Organometallics 2001, 20, 811–817.
- 4
- 4a F. Meyer, P. Rutsch, Chem. Commun. 1998, 1037–1038;
- 4b
F. Meyer, E. Kaifer, P. Kircher, K. Heinze, H. Pritzkow, Chem. Eur. J. 1999, 5, 1617–1630;
10.1002/(SICI)1521-3765(19990503)5:5<1617::AID-CHEM1617>3.0.CO;2-B CAS Web of Science® Google Scholar
- 4c
J. Ackermann, F. Meyer, E. Kaifer, H. Pritzkow, Chem. Eur. J. 2002, 8, 247–258.
10.1002/1521-3765(20020104)8:1<247::AID-CHEM247>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 5 J. C. Röder, F. Meyer, M. Konrad, E. Kaifer, H. Pritzkow, Eur. J. Org. Chem. 2001, 4479–4487.
- 6
- 6a M. E. Huttenloch, J. Diebold, U. Rief, H. H. Brintzinger, Organometallics 1992, 11, 3600–3607;
- 6b M. Enders, G. Kohl, H. Pritzkow, J. Organomet. Chem. 2001, 622, 66–73;
- 6c J. C. Röder, F. Meyer, R. F. Winter, E. Kaifer, J. Organomet. Chem. 2002, 641, 113–120.
- 7 J. C. Röder, F. Meyer, E. Kaifer, unpublished results.
- 8 The two CpMn(CO)2 moieties in 3− (and also in 3+) are vibrationally coupled, giving rise to a second (less intense) pair of IR bands. See also: C. G. Atwood, W. E. Geiger, J. Am. Chem. Soc. 1993, 115, 5310–5311.
- 9 Crystal structure of (K+ 3−)4⋅3.6 THF (C76H52K4Mn8N8O16⋅3.6 THF, Mr=2188.8): monoclinic, P21/c, a=23.071(5), b=25.404(5), c=15.501(3) Å, β=91.14(3), V=9083(3) Å3, Z=4, ρcalcd=1.601 g cm−3, μ(MoKα)=1.33 mm−1, 2θmax=54.9°, 20 751 independent reflections (Rint=0.093), 12 009 observed [I>2σ(I)], 1192 parameters; final R1[I>2σ(I)]=0.076, wR2=0.127, goodness of fit on F 2=1.020, largest difference peak +0.73/−0.48 eÅ−3. Data were collected on a Nonius Kappa CCD diffractometer at 200 K using MoKα radiation (λ=0.71073 Å). The structure was solved by direct methods with the SHELXS-97 and refined with the SHELXL-97 programs. Non-hydrogen atoms were refined in anisotropic models. Hydrogen atoms were placed at calculated positions and allowed to ride on the atoms they are attached to. CCDC 162374 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 10 The average KC distance is 3.06 Å in CpK and 3.37 Å for Karene interactions:
- 10a R. E. Dinnebier, U. Behrens, F. Olbrich, Organometallics 1997, 16, 3855–3858;
- 10b D. L. Clark, J. C. Gordon, J. C. Huffman, R. L. Vincent-Hollis, J. G. Watkin, B. D. Zwick, Inorg. Chem. 1994, 33, 5903–5911.
- 11
Ru: J. R. Perera, M. J. Heeg, H. B. Schlegel, C. W. Winter, J. Am. Chem. Soc. 1999, 121, 4536–4537; K:
Z. Hu, S. M. Gorun, Inorg. Chem. 2000, 40, 667–671; Tl:
G. B. Deacon, E. E. Delbridge, C. M. Forsyth, B. W. Skelton, A. H. White, J. Chem. Soc. Dalton Trans. 2000, 745–751; Eu:
G. B. Deacon, A. Gitlits, P. W. Roesky, M. R. Bürgstein, K. C. Lim, B. W. Skelton, A. H. White, Chem. Eur. J. 2001, 7, 127–138; Ba:
10.1002/1521-3765(20010105)7:1<127::AID-CHEM127>3.0.CO;2-1 CAS PubMed Web of Science® Google ScholarA. Steiner, G. T. Lawson, B. Walfort, D. Leusser, D. Stalke, J. Chem. Soc. Dalton Trans. 2001, 219–221.
- 12 J. E. Cosgriff, G. B. Deacon, Angew. Chem. 1997, 109, 298–299; Angew. Chem. Int. Ed. Engl. 1997, 37, 286.
- 13 d(K1⋅⋅⋅C/O)=3.195/3.023 Å has been observed in K3[Mn3(CO12)]: W. Schatz, H.-P. Neumann, B. Nuber, B. Kanellakopulos, M. L. Ziegler, Chem. Ber. 1991, 124, 453–463.
- 14 Values versus the saturated calomel electrode (SCE). ipa/ipc≈1; ipc/v1/2≈constant.
- 15 R. R. Gagné, C. A. Koval, T. J. Smith, M. C. Cimlino, J. Am. Chem. Soc. 1979, 101, 4571–4580; all symbols have their usual meanings. kth is taken to be equal to the EPR lifetime at the coalescence temperature (5.5×108 s−1). Eth is supposed to not change in the given temperature range and adiabatic electron transfer is assumed. See also:
- 15a R. C. Long, D. N. Hendrickson, J. Am. Chem. Soc. 1983, 105, 1513–1521;
- 15b S. K. Dutta, S. B. Kumar, S. Bhattacharyya, E. R. T. Tiekink, M. Chaudhury, Inorg. Chem. 1997, 36, 4954–4960.
- 16
- 16a K. G. Caulton, Coord. Chem. Rev. 1981, 38, 1–43;
- 16b D. Sellmann, J. Müller, P. Hofmann, Angew. Chem. 1982, 94, 708–709; Angew. Chem. Int. Ed. Engl. 1982, 21, 691;
- 16c A. Winter, G. Huttner, L. Zsolnai, P. Kroneck, M. Gottlieb, Angew. Chem. 1984, 96, 986–987; Angew. Chem. Int. Ed. Engl. 1984, 23, 975.
- 17
- 17a R. Gross, W. Kaim, Inorg. Chem. 1986, 25, 4865–4870;
- 17b W. Kaim, R. Gross, Comments Inorg. Chem. 1988, 7, 269–285;
- 17c C. A. Atwood, W. E. Geiger, J. Am. Chem. Soc. 2000, 122, 5477–5485.



41:13<>1.0.co;2-h.cover.gif)
