A Zipper-like Supramolecular Double-Chain based on 1 D π-π Stacking Interactions: Synthesis and Crystal Structure of [Cu(phen)(C4H4O4)(H2O)]2 · C4H6O4 (phen = 1,10-phenanthroline)
Abstract
[Cu(C12H8N2)(C4H4O4)(H2O)]2 · C4H6O4 was prepared by the reaction of succinic acid, CuCl2 · 2 H2O, 1,10-phenanthroline (phen = C12H8N2), and Na2CO3 in a CH3OH–H2O solution. The crystal structure (triclinic, P 1 (no. 2), a = 7.493(1), b = 9.758(1), c = 13.517(1) Å; α = 68.89(1)°, β = 88.89(1)°, γ = 73.32(1)°, Z = 1, R = 0.0308, wR2 = 0.0799 for 3530 observed reflections (F 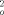 ≥ 2σ(F
≥ 2σ(F 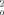 ) out of 3946 unique reflections) consists of hydrogen bonded succinic acid molecules and succinato bridged 1 D zipperlike supramolecular
) out of 3946 unique reflections) consists of hydrogen bonded succinic acid molecules and succinato bridged 1 D zipperlike supramolecular  [Cu(phen)(C4H4O4)2/2(H2O)]2 double chains based on 1 D π-π stacking interactions between the chelating phen systems at distances of 3.71 Å and 3.79 Å. The Cu atoms are fivefold trigonal bipyramidally coordinated by two N atoms of the bidentate chelating phen ligand and three O atoms of one water molecule and two bidentate bridging succinate ligands. The water O atom and one phen N atom are at the apical positions (equatorial: d(Cu–O) = 1.945, 2.254(2) Å, d(Cu–N) = 2.034(2) Å; axial: d(Cu–O) = 1.971(2) Å, d(Cu–N) = 1.995 Å).
[Cu(phen)(C4H4O4)2/2(H2O)]2 double chains based on 1 D π-π stacking interactions between the chelating phen systems at distances of 3.71 Å and 3.79 Å. The Cu atoms are fivefold trigonal bipyramidally coordinated by two N atoms of the bidentate chelating phen ligand and three O atoms of one water molecule and two bidentate bridging succinate ligands. The water O atom and one phen N atom are at the apical positions (equatorial: d(Cu–O) = 1.945, 2.254(2) Å, d(Cu–N) = 2.034(2) Å; axial: d(Cu–O) = 1.971(2) Å, d(Cu–N) = 1.995 Å).




