Sodium Dicarbonylcyclopentadienylferrate
Abstract
[12152-20-4; 12107-09-4] C7H5FeNaO2 (MW 199.96)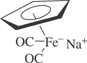
InChI = 1S/C5H5.2CO.Fe.Na/c1-2-4-5-3-1;2*1-2;;/h1-5H;;;;/q;;;-1;+1
InChIKey = DUBWVIJQCZHDKP-UHFFFAOYSA-N
InChI = 1S/C5H5.2CO.Fe.Na/c1-2-4-5-3-1;2*1-2;;/h1-5H;;;;/q;;;-1;+1
InChIKey = DUBWVIJQCZHDKP-UHFFFAOYSA-N
(nucleophilic source of iron;1 precursor to iron alkene complexes;1 precursor to η1-allyliron and η1-propargyliron complexes; precursor to acyliron complexes;2 precursor to iron carbenes;3 with subsequent oxidation will carbonylate alkyl halides or sulfonates4)
Solubility: sol ethereal organic solvents (Et2O, THF).
Preparative Methods: to Mercury (70 mL) stirring under nitrogen in a 500 mL flask with a stopcock in the bottom is slowly added Sodium metal (7.2 g), cut into small pieces, under a strong flow of nitrogen. The resulting amalgam is cooled to room temperature, and THF (100 mL) is added, followed by Bis(dicarbonylcyclopentadienyliron) (35.4 g, 0.1 mol). The mixture is stirred for 40 min, and the mercury is drained through the stopcock. The resulting Na[Fe(CO)2Cp] is used without further purification.5 An alternative preparation using sodium dispersion in THF at reflux has also been reported.6
Handling, Storage, and Precautions: generally prepared and used immediately; it is destroyed by protic solvents.



