Allyl Alcohol
Sylvain Taillemaud
Université de Montréal, Montréal, QC, Canada
Search for more papers by this authorSylvain Taillemaud
Université de Montréal, Montréal, QC, Canada
Search for more papers by this authorAbstract
[107-18-6] C3H6O1 (MW 58.04)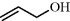
Key: common building block and allylation reagent
Physical Data: bp 98 °C.
Form Supplied in: colorless liquid.
Analysis of Reagent Purity: NMR spectroscopy, IR.
Preparative Method: allyl alcohol can be synthesized starting from glycerol using formic acid under heating.1
Purification: after being dried with potassium carbonate, calcium sulfate, or by azeotropic distillation of the contaminating water with benzene, allyl alcohol can be purified via fractional distillation over magnesium to be obtained free of peroxides.2
Bibliography
- 1
Kamm, O.;
Marvel, C. S.,
Org. Synth.
1921,
1,
15.
10.15227/orgsyn.001.0015 Google Scholar
- 2 Armarego, W. L. F. In Purification of Laboratory Chemicals, 6th Ed.; Butterworth-Heinemann: Oxford, 2009, p 98.
- 3 Jeffery, T., Tetrahedron Lett. 1991, 32, 2121.
- 4 Kang, S.-K.; Lee, H.-W.; Jang, S.-B.; Kim, T.-H.; Pyun, S.-J., J. Org. Chem. 1996, 61, 2604.
- 5 Chen, M.; Wang, J.; Chai, Z.; You, C.; Lei, A., Adv. Synth. Catal. 2012, 354, 341.
- 6 Masuyama, Y.; Takahara, J. P.; Kurusu, Y., J. Am. Chem. Soc. 1988, 110, 4473.
- 7 Takahara, J. P.; Masuyama, Y.; Kurusu, Y., J. Am. Chem. Soc. 1992, 114, 2577.
- 8 Araki, S.; Kamei, T.; Hirashita, T.; Yamamura, H.; Kawai, M., Org. Lett. 2000, 2, 847.
- 9 Kimura, M.; Shimizu, M.; Shibata, K.; Tazoe, M.; Tamaru, Y., Angew. Chem., Int. Ed. 2003, 42, 3392.
- 10 Zhu, S.-F.; Yang, Y.; Wang, L.-X.; Liu, B.; Zhou, Q.-L., Org. Lett. 2005, 7, 2333.
- 11 Ozawa, F.; Okamoto, H.; Kawagishi, S.; Yamamoto, S.; Minami, T.; Yoshifuji, M., J. Am. Chem. Soc. 2002, 124, 10968.
- 12 Kinoshita, H.; Shinokubo, H.; Oshima, K., Org. Lett. 2004, 6, 4085.
- 13 Lang, S. B.; Locascio, T. M.; Tunge, J. A., Org. Lett. 2014, 16, 4308.
- 14 Manabe, K.; Kobayashi, S., Org. Lett. 2003, 5, 3241.
- 15 Jiang, G.; List, B., Angew. Chem., Int. Ed. 2011, 50, 9471.
- 16 Zhou, H.; Zhang, L.; Xu, C.; Luo, S., Angew. Chem., Int. Ed. 2015, 54, 12645.
- 17 Pupo, G.; Properzi, R.; List, B., Angew. Chem., Int. Ed. 2016, 55, 6099.
- 18 Grenning, A. J.; Tunge, J. A., J. Am. Chem. Soc. 2011, 133, 14785.
- 19 Suzuki, Y.; Sun, B.; Sakata, K.; Yoshino, T.; Matsunaga, S.; Kanai, M., Angew. Chem., Int. Ed. 2015, 54, 9944.
- 20 Bunno, Y.; Murakami, N.; Suzuki, Y.; Kanai, M.; Yoshino, T.; Matsunaga, S., Org. Lett. 2016, 18, 2216.
- 21 Kumar, G. S.; Kapur, M., Org. Lett. 2016, 18, 1112.
- 22 Peralta-Hernandez, E.; Cortezano-Arellano, O.; Cordero-Vargas, A., Tetrahedron Lett. 2011, 52, 6899.
- 23 Liang, H.; Ito, S.; Yoshifuji, M., Org. Lett. 2004, 6, 425.
- 24 Banerjee, D.; Jagadeesh, R. V.; Junge, K.; Junge, H.; Beller, M., Angew. Chem., Int. Ed. 2012, 51, 11556.
- 25 Tsupova, S.; Maeeorg, U., Org. Lett. 2013, 15, 3381.
- 26 Kang, K.; Kim, J.; Lee, A.; Kim, W. Y.; Kim, H., Org. Lett. 2016, 18, 616.
- 27 Coudray, L.; Bravo-Altamirano, K.; Montchamp, J.-L., Org. Lett. 2008, 10, 1123.
- 28 Ramanathan, B.; Odom, A. L., J. Am. Chem. Soc. 2006, 128, 9344.
- 29 (a) Utsunomiya, M.; Miyamoto, Y.; Lpposhi, J.; Ohshima, T.; Mashima, K., Org. Lett. 2007, 9, 3371. (b) Ohshima, T.; Miyamoto, Y.; Ipposhi, J.; Nakahara, Y.; Utsunomiya, M.; Mashima, K., J. Am. Chem. Soc. 2009, 131, 14317.
- 30 Mukherjee, P.; Widenhoeferst, R. A., Org. Lett. 2010, 12, 1184.
- 31 McDonald, R. I.; Wong, G. W.; Neupane, R. P.; Stahl, S. S.; Landis, C. R., J. Am. Chem. Soc. 2010, 132, 14027.
- 32 Lightburn, T. E.; De Paolis, O. A.; Cheng, K. H.; Tan, K. L., Org. Lett. 2011, 13, 2686.
- 33 Crich, D.; Huang, X.; Newcomb, M., J. Org. Chem. 2000, 65, 523.
- 34 Jahn, U.; Rudakov, D., Synlett 2004, 1207.
- 35 Gonzalez, J.; Gonzalez, J.; Perez-Calleja, C.; Lopez, L. A.; Vicente, R., Angew. Chem., Int. Ed. 2013, 52, 5853.
- 36 Jones, R. J.; Rapoport, H., J. Org. Chem. 1990, 55, 1144.
- 37 Barluenga, J.; Vazquez-Villa, H.; Ballesteros, A.; Gonzalez, J. M., Org. Lett. 2002, 4, 2817.
- 38 Schneider, C.; Sreekanth, A. R.; Mai, E., Angew. Chem., Int. Ed. 2004, 43, 5691.
- 39 Yu, X.-Q.; Yoshimura, F.; Ito, F.; Sasaki, M.; Hirai, A.; Tanino, K.; Miyashita, M., Angew. Chem., Int. Ed. 2008, 47, 750.
- 40 Peschiulli, A.; Gun'ko, Y.; Connon, S. J., J. Org. Chem. 2008, 73, 2454.
- 41 Berkessel, A.; Cleemann, F.; Mukherjee, S.; Mueller, T. N.; Lex, J., Angew. Chem., Int. Ed. 2005, 44, 807.
- 42 Ireland, R. E.; Norbeck, D. W., J. Am. Chem. Soc. 1985, 107, 3279.
- 43 RajanBabu, T. V., J. Org. Chem. 1988, 53, 4522.
- 44
Chen, G.-W.;
Kirschning, A.,
Chem. Eur. J.
2002,
8,
2717.
10.1002/1521-3765(20020617)8:12<2717::AID-CHEM2717>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 45 Cui, J.-N.; Teraoka, R.; Ema, T.; Sakai, T.; Utaka, M., Tetrahedron Lett. 1997, 38, 3021.
- 46 Tosi, G.; Zironi, F.; Caselli, E.; Forni, A.; Prati, F., Synthesis 2004, 1625.
- 47 Bertau, M., Tetrahedron Lett. 2001, 42, 1267.
- 48 Usui, Y.; Sato, K.; Tanaka, M., Angew. Chem., Int. Ed. 2003, 42, 5623.
- 49 Huynh, M. H. V.; Witham, L. M.; Lasker, J. M.; Wetzler, M.; Mort, B.; Jameson, D. L.; White, P. S.; Takeuchi, K. J., J. Am. Chem. Soc. 2003, 125, 308.
- 50 Nishiyama, Y.; Tanimizu, H.; Tomita, T., Tetrahedron Lett. 2007, 48, 6405.
- 51 Llaveria, J.; Beltrán Á; Sameera, W. M. C.; Locati, A.; Díaz-Requejo, M. M.; Matheu, M. I.; Castillón, S.; Maseras, F.; Pérez, P. J., J. Am. Chem. Soc. 2014, 136, 5342.
- 52 Tsuji, J.; Minami, I., Acc. Chem. Res. 1987, 20, 140
- 53 Behenna, D. C.; Stoltz, B. M., J. Am. Chem. Soc. 2004, 126, 15044.
- 54 (a) Guibé, F., Tetrahedron 1997, 53, 13509. (b) Guibé, F., Tetrahedron 1998, 54, 2967.



