Allylcyclopentadienyl[(4R,5R)- and (4S,5S)-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanolato-O,O′]-titanium
Abstract
[139354-61-3] C39H38O4Ti (MW 618.23) InChIKey = QKHHREYDRUIQSO-HVVJQNAGSA-N [139354-59-4] C39H38O4Ti (MW 618.23) InChIKey = QKHHREYDRUIQSO-CRMRIEQBSA-N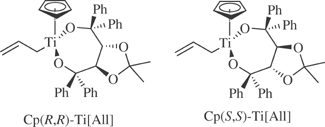
(reagent for the asymmetric allyltitanation of aldehydes to produce homoallylic alcohols)2
Solubility: used as the crude preparation in Et2O.
Analysis of Reagent Purity: 1H NMR and 13C NMR (CD2Cl2).2
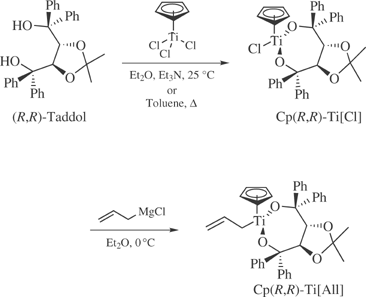
Preparative Methods: it can be prepared in two steps from either (4R,trans)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol (R,R-Taddol)3 or (4S,trans)-2,2-dimethyl-α,α,α′,α′-tetraphenyl-1,3-dioxolane-4,5-dimethanol (S,S-Taddol)3 (eq 1).2
Handling, Storage, and Precautions: best handled as stock solution in Et2O (ca. 0.82 M) which must be protected from moisture. Should be used just after preparation. Reactions should be carried out in dry equipment and with absolute solvents under Ar or N2.