Aminoiminomethanesulfonic Acid
Abstract
[1184-90-3] C1H4N2O3S (MW 124.13)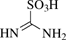
InChI = 1S/CH4N2O3S/c2-1(3)7(4,5)6/h(H3,2,3)(H,4,5,6)
InChIKey = AOPRFYAPABFRPU-UHFFFAOYSA-N
(parent compound and its derivatives guanylate amines; some derivatives give triazoles with azide and aminoiminoethanenitriles with cyanide as nucleophile)
Alternate Name: formamidinesulfonic acid.
Physical Data: mp 131–131.5 °C when highly pure; around 125 °C with dec before purification.
Solubility: sol water; slightly sol methanol, ethanol; insol ether.
Preparative Methods: by the oxidation of thiourea or aminoiminomethanesulfinic acid (formamidinesulfinic acid) with Peracetic Acid. Many substituted aminoiminomethanesulfonic acids can be prepared in the same way.1, 2 Others have utilized
Purification: recrystallize from glacial acetic acid.
Handling, Storage, and Precautions: stable for at least a few weeks at room temperature. After drying, it remains stable for at least 5 months if kept in a freezer. Thiourea and its metabolites (probably oxidized thiourea) are tumorigenic and cause lung edema. All direct contact with the compound should be avoided; for example, a dust mask should be worn. All residues should be destroyed with strong bleach solution. Many substituted thioureas and their metabolites are also biologically active. Use in a fume hood.



