Alumina†
Alan S. Florjancic
The Ohio State University, Columbus, OH, USA
Search for more papers by this authorAlan S. Florjancic
The Ohio State University, Columbus, OH, USA
Search for more papers by this authorAbstract
[1344-28-1] Al2O3 (MW 101.96)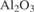
InChI = 1S/2Al.3O
InChIKey = TWNQGVIAIRXVLR-UHFFFAOYSA-N
(a mildly acidic, basic, or neutral support for chromatographic separations; a reagent for catalyzing dehydration, elimination, addition, condensation, epoxide opening, oxidation, and reduction reactions)
Alternate Name: γ-alumina.
Physical Data: mp 2015 °C; bp 2980 °C; d 3.97 g cm−3.
Solubility: slightly sol acid and alkaline solution.
Form Supplied in: fine white powder, widely available in varying particle size (50–200 μm; 70–290 mesh), in acidic (pH 4), basic (pH 10), and neutral (pH 7) forms.
Drying: the activity of alumina has been classified by the Brockmann scale into five grades. The most active form, grade I, is obtained by heating alumina to 200 °C while passing an inert gas through the system, or heating to ∼400 °C in an open vessel, followed by cooling in a dessicator. Addition of 3–4% (w/w) water and mixing for several hours converts grade I alumina to grade II. Other grades are similarly obtained (grade III, 5–7%; grade IV, 9–11%; grade V, 15–19% water).2, 3
Handling, Storage, and Precautions: inhalation of fine mesh alumina can cause respiratory difficulties. Alumina is best handled under a fume hood and stored under dry, inert conditions.
Bibliography
- 1 Posner, G. H. Angew. Chem. Int. Ed. Engl. 1978, 17, 487.
- 2 Perrin, D. D.; Armarego, W. L. F. Purification of Laboratory Chemicals; Pergamon: New York, 1988; pp 20, 310.
- 3 (a) Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. Vogel's Textbook of Practical Organic Chemistry; Longman-Wiley: New York, 1989; p 212. (b) Fieser & Fieser 1967, 1, 19.
- 4 Knözingery, H. Angew. Chem. Int. Ed. Engl. 1968, 7, 791.
- 5(a) Hershberg, E. B.; Ruhoff, J. R. Org. Synth. 1937, 17, 25. (b) Newton, L. W.; Coburn, E. R. Org. Synth., Coll. Vol 1955, 3, 312. (c) Sawyer, R. L.; Andrus, D. W. Org. Synth., Coll. Vol 1955, 3, 276.
- 6(a) von Rudloff, E. Can. J. Chem. 1961, 39, 1860. (b) Corey, E. J.; Hortmann, A. G. J. Am. Chem. Soc. 1965, 87, 5736. (c) Barrett, H. C.; Büchi, G. J. Am. Chem. Soc. 1967, 89, 5665.
- 7(a) Kobayashi, S.; Shinya, M.; Taniguchi, H. Tetrahedron 1971, 71. (b) Ishii, H.; Tozyo, T.; Nakamura, M.; Funke, E. Chem. Pharm. Bull. 1972, 20, 203. (c) Gotthardt, H.; Hammond, G. S. Ber. Dtsch. Chem. Ges./Chem. Ber. 1974, 107, 3922. (d) Mayr, H.; Huisgen, R. Angew. Chem. Int. Ed. Engl. 1975, 14, 499. (e) Posner, G. H.; Gurria, G. M.; Babiak, K. A. J. Org. Chem. 1977, 42, 3173. (f) Vidal, J.; Huet, F. Tetrahedron 1986, 27, 3733.
- 8(a) Strobach, D. R.; Boswell, G. A., Jr., J. Org. Chem. 1971, 36, 818. (b) Boswell, G. A., Jr. J. Org. Chem. 1966, 31, 991.
- 9(a) Miller, R. B.; McGarvey, G. J. Org. Chem. 1978, 43, 4424. (b) Miller, R. B.; McGarvey, G. Synth. Commun. 1977, 7, 475.
- 10 Sponholtz, W. R., III; Pagni, R. M.; Kabalka, G. W.; Green, J. F.; Tan, L. C. J. Org. Chem. 1991, 56, 5700.
- 11 Cava, M. P.; Pollack, N. M.; Mamer, O. A.; Mitchell, M. J. J. Org. Chem. 1971, 36, 3932.
- 12 Labar, D.; Hevesi, L.; Dumont, W.; Krief, A. Tetrahedron 1978, 1141.
- 13(a) Bourns, A. N.; Embleton, H. W.; Hansuld, M. K. Org. Synth., Coll. Vol, 1963, 4, 795. (b) Glacet, C.; Adrian, G. C.R. Hebd. Seances Acad. Sci., Ser. C 1969, 269, 1322.
- 14(a)
Texier,-Boullet, F.;
Klein, B.;
Hamelin, J.
Synthesis
1986,
409.
10.1055/s-1986-31655 Google Scholar(b) Tolstikov, G. A.; Galin, F. Z.; Makaev, F. Z. Zh. Org. Khim. 1989, 25, 875.
- 15(a)
LeBlanc, R. J.;
Vaughan, K.
Can. J. Chem.
1972,
50,
2544.
(b)
Higashino, T.;
Suzuki, K.;
Hayashi, E.
Chem. Pharm. Bull.
1978,
26,
3485.
(c)
Bladé-Font, A.
Tetrahedron
1980,
21,
2443.
(d)
Hooper, D. L.;
Manning, H. W.; LaFrance, R. J.;
Vaughan, K.
Can. J. Chem.
1986,
65,
250.
10.1139/v86-043 Google Scholar(e) Hull, J. W., Jr.; Otterson, K.; Rhubright, D. J. Org. Chem. 1993, 58, 520.
- 16 Kamitori, Y.; Hojo, M.; Masuda, R.; Yoshida, T. Tetrahedron 1985, 26, 4767.
- 17 McPhail, A. T.; Onan, K. D. Tetrahedron 1973, 4641.
- 18(a) Pelletier, S. W.; Venkov, A. P.; Finer-Moore, J.; Mody, N. V. Tetrahedron 1980, 21, 809. (b) Pelletier, S. W.; Gebeyehu, G.; Mody, N. V. Heterocycles 1982, 19, 235. (c) Dzurilla, M.; Kutschy, P.; Kristian, P. Synthesis 1985, 933.
- 19 Pagni, R.; Kabalka, G. W.; Boothe, R.; Gaetano, K.; Stewart, L. J.; Conaway, R.; Dial, C.; Gray, D.; Larson, S.; Luidhardt, T. J. Org. Chem. 1988, 53, 4477.
- 20 Kropp, P. J.; Daus, K. A.; Tubergen, M. W.; Kepler, K. D.; Wilson, V. P.; Craig, S. L.; Baillargeon, M. M.; Breton, G. W. J. Am. Chem. Soc. 1993, 115, 3071, and references cited therein. Addition of HN3: Breton, G. W.; Daus, K. A.; Kropp, P. J. J. Org. Chem. 1992, 57, 6646.
- 21(a) Rosan, A.; Rosenblum, M. J. Org. Chem. 1975, 40, 3621. (b) Texier-Boullet, F.; Foucaud, A. Tetrahedron 1982, 23, 4927. (c) Rosini, G.; Ballini, R.; Sorrenti, P. Synthesis 1983, 1014. (d) Varma, R. S.; Kabalka, G. W.; Evans, L. T.; Pagni, R. M. Synth. Commun. 1985, 15, 279. (e) Nesi, R.; Stefano, C.; Piero, S.-F. Heterocycles 1985, 23, 1465. (f) Rosini, G.; Ballini, R.; Petrini, M.; Sorrenti, P. Synthesis 1985, 515. (g) Foucaud, A.; Bakouetila, M. Synthesis 1987, 854. (h) Moison, H.; Texier-Boullet, F.; Foucaud, A. Tetrahedron 1987, 43, 537.
- 22(a) Rosini, G.; Marotta, E.; Ballini, R.; Petrini, M. Synthesis 1986, 237. (b) Ballini, R.; Petrini, M.; Marcantoni, E.; Rosini, G. Synthesis 1988, 231.
- 23 Texier-Boullet, F.; Villemin, D.; Ricard, M.; Moison, H.; Foucaud, A. Tetrahedron 1985, 41, 1259.
- 24 Isoxazoline: Rosini, G.; Galarini, R.; Marotta, E.; Righi, P. J. Org. Chem. 1990, 55, 781. Rosini, G.; Marotta, E.; Righi, E.; Seerden, J. P. J. Org. Chem. 1991, 56, 6258.
- 25(a) Parlar, H.; Baumann, R. Angew. Chem. Int. Ed. Engl. 1981, 20, 1014 (b) Koreeda, M.; Ricca, D. J.; Luengo, J. I. J. Org. Chem. 1988, 53, 5586.
- 26(a) Tietze, L. F.; Beifuss, U.; Ruther, M. J. Org. Chem. 1989, 54, 3120. (b) Tietze, L. F.; Beifuss, U. Synthesis 1988, 359.
- 27 Pogrebnoi, S. I.; Kalyan, Y. B.; Krimer, M. Z.; Smit, W. A. Tetrahedron 1987, 28, 4893.
- 28 Cullinane, N. M.; Chard, S. J.; Dawkins, C. W. C. Org. Synth., Coll. Vol 1963, 4, 520.
- 29 Baird, R.; Winstein, S. J. Am. Chem. Soc. 1963, 85, 567.
- 30 Villemin, D. Chem. Commun./J. Chem. Soc., Chem. Commun. 1985, 870.
- 31(a) Ogawa, H.; Chihara, T.; Teratani, S.; Taya, K. Bull. Chem. Soc. Jpn. 1986, 59, 2481. (b) Cooke, F.; Magnus, P. Chem. Commun./J. Chem. Soc., Chem. Commun. 1976, 519.
- 32 Hart, P. A.; Sandmann, R. A. Tetrahedron 1969, 305.
- 33 Ogawa, H.; Chihara, T.; Taya, K. J. Am. Chem. Soc. 1985, 107, 1365.
- 34
Sarratosa, F.
J. Chem. Educ.
1964,
41,
564.
10.1021/ed041p564 Google Scholar
- 35(a) Posner, G. H.; Rogers, D. Z. J. Am. Chem. Soc. 1977, 99, 8208. (b) Posner, G. H.; Rogers, D. Z. J. Am. Chem. Soc. 1977, 99, 8214. (c) Evans, D. A.; Golob, A. M.; Mandel, N. S.; Mandel, G. S. J. Am. Chem. Soc. 1978, 100, 8170.
- 36 Kropf, H.; Amirabadi, H. M.; Mosebach, M.; Torkler, A.; von Wallis, H. Synthesis 1983, 587.
- 37 Hudrlik, P. F.; Hudrlik, A. M.; Kulkarni, A. K. Tetrahedron 1985, 26, 139.
- 38(a) Boeckman, R. K., Jr.; Bruza, K. J.; Heinrich, G. R. J. Am. Chem. Soc. 1978, 100, 7101. (b) Niwa, M.; Iguchi, M.; Yamamura, S. Tetrahedron 1979, 4291.
- 39(a) Dhillon, R. S.; Chhabra, B. R.; Wadia, M. S.; Kalsi, P. S. Tetrahedron 1974, 401. (b) Antonioletti, R.; D'Auria, M.; de Mico, A.; Piancatelli, G.; Scettri, A. Tetrahedron 1983, 39, 1765.
- 40(a) Tsuboi, S.; Furutani, H.; Takeda, A. Synthesis 1987, 292. (b) Harigaya, Y.; Yotsumoto, K.; Takamatsu, S.; Yamaguchi, H.; Onda, M. Chem. Pharm. Bull. 1981, 29, 2557.
- 41(a) Posner, G. H.; Perfetti, R. B.; Runquist, A. W. Tetrahedron 1976, 3499. (b) Posner, G. H.; Chapdelaine, M. J. Synthesis 1977, 555. (c) Posner, G. H.; Chapdelaine, M. J. Tetrahedron 1977, 3227.
- 42 Posner, G. H.; Runquist, A. W.; Chapdelaine, M. J. J. Org. Chem. 1977, 42, 1202. Also see: Suginome, H.; Kato, K. Tetrahedron 1973, 4143.
- 43(a) Pan, H.-L.; Cole, C.-A.; Fletcher, T. L. Synthesis 1975, 716. (b) Liu, K.-T.; Tong, Y.-C. Synthesis 1978, 669.
- 44
Review:
Laszlo, P.
Comprehensive Organic Synthesis
1991,
7,
839.
Recent examples:
10.1016/B978-0-08-052349-1.00215-8 Google Scholar(a) Singh, S.; Dev, S. Tetrahedron 1993, 49, 10959. (b) Lee, D. G.; Chen, T.; Wang, Z. J. Org. Chem. 1993, 58, 2918. (c) Morimoto, T.; Hirano, M.; Iwasaki, K.; Ishikawa, T. Chem. Lett. 1994, 53. (d) Santaniello, E.; Ponti, F.; Manzocchi, A. Synthesis 1978, 891.
- 45 Soai, K.; Watanabe, M.; Yamamoto, A. J. Org. Chem. 1990, 55, 4832.
- 46(a) Craig, J. C.; Naik, A. R. J. Am. Chem. Soc. 1962, 84, 3410. (b) Gonzalez, A.; Galvez, C. Synthesis 1982, 946. (c) Luh, T.-Y.; Chow, H.-F.; Leung, W. Y.; Tam, S. W. Tetrahedron 1985, 41, 519. (d) Nagano, H.; Masunaga, Y.; Matsuo, Y.; Shiota, M. Bull. Chem. Soc. Jpn. 1987, 60, 707. See also: (e) Métayer, A.; Barbier, M. Bull. Soc. Claim. Fr. 1972, 3625.
- 47(a) Marshall, J. A.; Roebke, H. J. Org. Chem. 1966, 31, 3109. (b) Hudlicky, T.; Srnak, T. Tetrahedron 1981, 22, 3351. (c) Reetz, M. T.; Wenderoth, B.; Urz, R. Ber. Dtsch. Chem. Ges./Chem. Ber. 1985, 118, 348. (d) Hatzigrigoriou, E.; Schmitt, M.-C.; Wartski, L. Tetrahedron 1988, 44, 4457. Also see: (e) Scettri, A.; Piancatelli, G.; D'Auria, M.; David, G. Tetrahedron 1979, 35, 135.
- 48(a) Larock, R. C.; Chow, M.-S.; Smith, S. J. J. Org. Chem. 1986, 51, 2623. (b) Manning, D. T.; Coleman, H. A. J. J. Org. Chem. 1969, 34, 3248.
- 49 Tsuboi, S.; Matsuda, T.; Mimura, S.; Takeda, A. Org. Synth., Coll. Vol 1993, 8, 251.
- 50 Johns, W. F.; Jerina, D. M. J. Org. Chem. 1963, 28, 2922.
- 51 Boar, R. B.; McGhie, J. F.; Robinson, M.; Barton, D. H. R.; Horwell, D. C.; Stick, R. V. J. Chem. Soc., Perkin Trans. 1 1975, 1237.
- 52 Coutts, I. G. C.; Culbert, N. J.; Edward, M.; Hadfield, J. A.; Musto, D. R.; Pavlidis, V. H.; Richards, D. J. J. Chem. Soc., Perkin Trans. 1 1985, 1829.
- 53(a) Greene, A. E.; Cruz, A.; Crabbé, P. Tetrahedron 1976, 2707. (b) van Leusen, A. M.; Strating, J. Org. Synth., Coll. Vol 1988, 6, 981.
- 54(a) Posner, G. H.; Oda, M. Tetrahedron 1981, 22, 5003. (b) Rana, S. S.; Barlow, J. J.; Matta, K. L. Tetrahedron 1981, 22, 5007. (c) Posner, G. A.; Okada, S. S.; Babiak, K. A.; Miura, K.; Rose, R. K. Synthesis 1981, 789. (d) Nagasawa, K.; Yoshitake, S.; Amiya, T.; Ito, K. Synth. Commun. 1990, 20, 2033.



