Allyltitanium Triphenoxide†
Abstract
[130004-39-6] C21H20O3Ti (MW 368.27)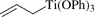
InChI = 1S/3C6H6O.C3H5.Ti/c3*7-6-4-2-1-3-5-6;1-3-2;/h3*1-5,7H;3H,1-2H2;/q;;;;+3/p-3
InChIKey = WLDVUFJBKRSUBE-UHFFFAOYSA-K
(reagent for the regioselective allylation of epoxides at the most substituted carbon;2 the crotyl homolog reacts stereoselectively with aldehydes3 and ketones4)
Solubility: reactions are normally performed in THF.
Form Supplied in: not available commercially.
Preparative Methods: the reagent is prepared in situ by reacting the corresponding Grignard derivative in THF at −78 °C → −30 °C with ClTi(OPh)3.3 The latter is prepared by refluxing Chlorotitanium Triisopropoxide and phenol in toluene, with the azeotropic removal of isopropanol. Reactions of the reagent with epoxides are normally performed at −78 °C, while reactions with aldehydes and ketones are started at −100 °C.



