O-Allylhydroxylamine
Abstract
[6542-54-7] C3H7NO (MW 73.11)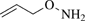
InChI = 1S/C3H7NO/c1-2-3-5-4/h2H,1,3-4H2
InChIKey = KPTCZURLWZSRKB-UHFFFAOYSA-N
(O-allyloximes, pyridine synthesis)
Alternate Name: O-2-propenylhydroxylamine.
Physical Data: bp 80 °C, IR 3350 cm−1. The reagent is typically prepared as the hydrochloride salt which is a white solid that may be recrystallized from EtOH and ether.
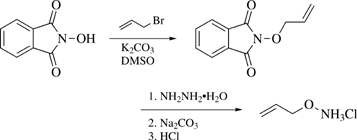
Preparative Methods: Allyl Bromide (39 mL, 0.46 mol) is added dropwise with stirring to a suspension of anhydrous K2CO3 (43 g) and commercially available N-hydroxyphthalimide (50 g, 0.31 mol) in DMSO (500 mL) at 25 °C. After addition, the mixture is stirred at room temp for 24 h and then poured into cold water (3 L). The precipitate is collected, washed with water, and dried. Recrystallization from EtOH gives N-allyloxyphthalimide (87%), mp 56–57 °C. A mixture of N-allyloxyphthalimide (10 g, 0.05 mol), hydrazine hydrate (5 mL, 0.1 mol), and EtOH (100 mL) is refluxed for 2 h and then cooled and poured into 3% aqueous Na2CO3 (500 mL). The solution is extracted with ether. The ether extract is washed with water and then 5 mL of conc HCl are added. Evaporation gives a solid which is recrystallized from EtOH and ether to afford O-allylhydroxylamine hydrochloride as a white solid (eq 1). The free amine can be liberated by distillation from KOH (bp 80 °C)1 or by treatment with ammonia in situ.2 It may be conveniently stored as the N-allyloxphthalimide intermediate which is converted to O-allylhydroxylamine hydrochloride as needed.
Handling, Storage, and Precautions: use in a fume hood.