2-aryl-1,3-dithianes and -dithiolanes: A nearly ideal series for relating the energies for bond breaking to electron transfer
Daniel T. Stoelting
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346
Search for more papers by this authorRichard T. Ludwig
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346
Search for more papers by this authorCorresponding Author
Edward M. Arnett
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346Search for more papers by this authorDaniel T. Stoelting
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346
Search for more papers by this authorRichard T. Ludwig
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346
Search for more papers by this authorCorresponding Author
Edward M. Arnett
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346
Department of Chemistry, Duke University, Box 90346, Durham, NC 27708-0346Search for more papers by this authorAbstract
By virtue of the stabilizing effect of the 1,3-sulfur atoms on the carbocations, radicals, and carbanions generated from the title compounds, it has been possible to measure a variety of bond-making and bond-breaking processes in the two very similar solvents, DMSO and sulfolane, and relate them to electron-transfer energies obtained by electrochemical techniques. Important properties reported from this and previously published work are as follows: heats of hydride transfer to the cations from cyanoborohydride ion, pK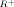 in aqueous acid, heats of deprotonation by K+ DMSYL/DMSO, pKHA, redox potentials for the cations, and carbanions, which relate their energies to their conjugate radicals and to each other. The results support our previous assertion that the electron-transfer energy between the three trivalent oxidation states of carbon and the Parr-Pearson absolute hardness, ϵ, derived from it are the fundamental properties that determine energies for making and breaking two-electron bonds and thus determine most of organic chemistry.
in aqueous acid, heats of deprotonation by K+ DMSYL/DMSO, pKHA, redox potentials for the cations, and carbanions, which relate their energies to their conjugate radicals and to each other. The results support our previous assertion that the electron-transfer energy between the three trivalent oxidation states of carbon and the Parr-Pearson absolute hardness, ϵ, derived from it are the fundamental properties that determine energies for making and breaking two-electron bonds and thus determine most of organic chemistry.
Excellent correlations are found for the substituent effects on energy changes associated with the various processes for making and breaking bonds to the cations, radicals, and carbanions and the electron-transfer energies for interconverting them. Many comparisons can be made with the corresponding 2-aryl-1,3-dioxo systems. Careful “bookkeeping” of these energies through appropriate thermochemical cycles shows excellent consistency despite a small solvent effect for transferring the ions from sulfolane to DMSO.
Direct reaction of the carbocation with the carbanion of 2-phenyl-1,3-dithiane produced a clean formation of the dimer from which the heat of heterolysis (40.6 kcal/mol) and homolysis (19.1 kcal/mol) could be calculated.
AM1 structures and heats of formation of two neutral species and two cations, a radical and an anion, have been computed and are generally consistent with stabilizing interactions of the gem sulfurs with the reactive center.
The present study is the first, to our knowledge, to provide a coordinated view of the energies for generating the carbocations, radicals, and carbanions from a series of heterocycles. These energies are related to each other and to the electron-transfer energies for interconverting these reactive trivalent forms of carbon. © 1996 John Wiley & Sons, Inc.
References
- 1(a) E. M. Arnett, R. A. Flowers II, A. E. Meekhof, L. Miller, J. Am. Chem. Soc., 115, 1993, 12603–12604. (b) E. M. Arnett, R. A. Flowers II, R. T. Ludwig, A. E. Meekoff, S. A. Walek, Pure Appl. Chem., 67, 1995, 729–734.
- 2 E. M. Arnett, R. A. Flowers II, R. T. Ludwig, A. E. Meekhof, S. Walek, (submitted).
- 3(a) G. A. Olah, P. von R. Schleyer: Carbonium Ions ( 4 vols.), Wiley-Interscience, New York, London, Sydney, Toronto (1970). (b) D. Bethell, V. Gold: Carbonium Ions, An Introduction, Academic Press, London, New York (1967). (c) C. D. Nenitzescu: in G. A. Olah, P. von R. Schleyer (eds): Carbonium Ions, Vol. 1, Interscience Publishers, New York (1968).
- 4(a) E. M. Arnett, R. A. Flowers II, A. E. Meekoff, A. Pourjavadi, S. A. Walek, J. Phys. Org. Chem., 7, 1994, 663–671. (b) R. F. Childs, R. M. Orgias, C. J. L. Lock, M. Mahendran, Can. J. Chem., 71, 1993, 836.
- 5 D. F. McMillen, D. M. Golden, Ann. Rev. Phys. Chem., 33, 1982, 493–532.
- 6 S. W. Benson: Thermochemical Kinetics, 2nd Ed., Wiley-Interscience Publication, New York (1976).
- 7(a) F. G. Bordwell, X.-M. Zhang, Acc. Chem. Res., 26, 1993, 510–517. (b) F. G. Bordwell, A. V. Satish, S. Zhang, X.-M. Zhang, Pure Appl. Chem., 735–740.
- 8(a) N. C. Deno, J. J. Jaruzelski, A. Schriesheim, J. Am. Chem. Soc., 77, 1955, 304 (b) N. C. Deno, A. Schriesheim, J. Am. Chem. Soc., 77, 1955, 3051. (c) N. C. Deno, W. L. Evans, J. Am. Chem. Soc., 79, 1957, 5804. (d) E. M. Arnett, R. D. Bushick, J. Am. Chem. Soc., 86, 1964, 1564.
- 9(a) W. S. Matthews, J. E. Bares, J. E. Bartmess, F. G. Bordwell, F. J. Cornforth, Z. Margolin, R. J. McCallum, G. J. McCollum, N. R. Vanier, J. Am. Soc., 97, 1975, 7006. (b) F. G. Bordwell, Acc. Chem. Res., 21, 1988, 456–463. (c) Cumulative printout of pKHA values as of February 1992 graciously provided by Prof. F. G. Bordwell.
- 10(a) E. M. Arnett, T. C. Moriarity, L. E. Small, J. P. Rudolph, R. P. Quirk, J. Am. Chem. Soc., 95, 1973, 1492. (b) E. M. Arnett, D. E. Johnston, L. E. Small, J. Am. Chem. Soc., 97, 1975, 5598. (c) E. M. Arnett, K. G. Venkatasubramanian, J. Org. Chem., 48, 1983, 1569.
- 11 Since the pKHA of sulfolane is about 31 (18 b) (DMSO, 35), it would be reasonable that this medium would be deprotonated rapidly by the very basic dioxolenyl anions formed at the electrode. This remains a moot point since the Ered2s of the dioxolenium cations appear to be reversible.
- 12(a) V. D. Parker, Acta Chem. Scand., 46, 1992, 1133–1134. (b) J.-P. Cheng, K. L. Handoo, V. D. Parker, J. Am. Chem. Soc., 115, 1993, 2655–2660.
- 13 B. A. Sim, P. H. Milne, D. Griller, D. D. M. Wayner, J. Am. Chem. Soc., 112, 1990, 6635–6638.
- 14 R. Breslow, Pure Appl. Chem., 40, 1974, 493–509.
- 15(a) E. M. Arnett, N. G. Harvey, K. Amarnath, J.-P. Cheng, J. Am. Chem. Soc., 111, 4143. (b) E. M. Arnett, K. Amarnath, N. G. Harvey, J.-P. Cheng, Science, 247, 1990, 42. (c) E. M. Arnett, R. A. Flowers II, Chem. Soc. Rev., 22, 1993, 9–15.
- 16 E. M. Arnett, K. Amarnath, N. G. Harvey, J.-P. Cheng, J. Am. Chem. Soc., 112, 344.
- 17 E. M. Arnett, K. Amarnath, N. G. Harvey, S. Venimadhavan, J. Am. Chem. Soc., 112 1990, 7346.
- 18 S. Venimadhavan, K. Amarnath, N. G. Harvey, E. M. Arnett, J. Am. Chem. Soc., 114, 1992, 221.
- 19 E. M. Arnett, S. Venimadhavan, K. Amarnath, J. Am. Chem. Soc., 11, 1992, 5.
- 20(a) R. G. Parr, R. G. Pearson, J. Am. Chem. Soc., 105, 1983, 7512. (b) R. G. Parr, W. Yang: Density Functional Theory of Atoms and Molecules Oxford University Press, New York (1989). (c) P. K. Chattaraj, H. Lee, R. G. Parr, J. Am. Chem. Soc., 113, 1991, 1855. (d) P. K. Chattaraj, R. G. Parr; Structure and Bonding, Vol. 80, Springer-Verlag, Heidelberg, pp. 11–26 (1993). (e) R. G. Parr, Z. Zhou, Acc. Chem. Res., 1993, 26. (f) P. K. Chattaraj, A. Cedillo, R. A. Parr, E. M. Arnett, J. Org. Chem., 60, 1995, 4707.
- 21(a) R. G. Pearson, J. Org. Chem., 52, 1987, 2131–2136. (b) R. G. Pearson, J. Org. Chem., 54, 1989, 1423. (c) R. G. Pearson, J. Am. Chem. Soc., 110, 1988, 7684. (d) R. G. Pearson, J. Mol. Struct. (Theochem), 255, 261–270. (e) R. G. Pearson, Acc. Chem. Res., 26, 1993, 250–255. (f) R. G. Pearson, J. Am. Chem. Soc., 108, 1986, 6109. (g) R. G. Pearson, Inorg. Chim. Acta, 240, 1995, 93. (h) R. G. Pearson, Int. J. Quant. Chem., 56(4), 1995, 211.
- 22 E. M. Arnett, R. T. Ludwig, J. Am. Chem. Soc., 117, 1995, 6627–6628.
- 23(a) See for example: R. E. Dessy, W. Kitching, T. Psarras, R. Salinger, A. Chen, T. Chivers, J. Am. Chem. Soc., 88, 1966, 460. (b) M. R. Feldman, W. C. Flythe, J. Org. Chem., 43, 1978, 596. (c) Y. Okamoto, J. Org. Chem., 39, 1983, 40. (d) D. D. M. Wayner, J. Am. Chem. Soc., 112, 1990, 6635. (e) E. M. Arnett, J. A. Harrelson, Gazzetta Chem. Ital., 117, 1987, 237. (f) V. D. Parker, J. Org. Chem., 58, 1993, 5811–5815.
- 24 E. M. Arnett, S. Venimadhavan, J. Am. Chem. Soc., 113, 1991, 6967.
- 25 J. P. Richard, Tetrahedron, 51, 1995, 1535–1573.
- 26 S. W. Benson, Angewandte Chem., 17, 1978, 812–819.
- 27(a) F. G. Bordwell, X.-M. Zhang, A. V. Satish, I.-P. Cheng, J. Am. Chem. Soc., 116, 1994 6605–6610. (b) K. B. Clark, D. D. M. Wayner, J. Am. Chem. Soc., 113, 1991, 9363–9365. (c) J. M. White, Aust. J. Chem., 48, 1995, 1227–1251. (d) P. von R. Schleyer, A. J. Kos, Tetrahedron, 39, 1983, 1141–1150. (e) L. A. Torres, A. Rojas, G. Cuevas, E. Juaristi, J. Phys. Org. Chem., 7, 1994, 561–5.
- 28(a) P. von R. Schleyer, E. D. Jemmis, G. W. Spitznagel, J. Am. Chem. Soc., 107, 1985, 6393–6394. (b) Y.-D. Wu, W. Kirmse, K. N. Houk, J. Am. Chem. Soc., 112, 1990, 4557–4559. (c) A. J. Kresge, M. Leibovitch, Can. J. Chem., 68, 1990, 1786–1790. (d) Y. Apeloig, M. Karni, J. Chem. Soc. Perkin Trans. II, 1988, 625–636. (e) E. Juaristi, J. Tapia, R. Mendez, Tetrahedron, 42, 1986, 1253–1264. (f) E. Juaristi, G. Cuevas, A. Vela, J. Am. Chem. Soc., 116, 1994, 5796–5804. (g) E. Juaristi, L. Valle, B. A. Valenzuela, M. A. Aguilar, J. Am. Chem. Soc., 1986, 2000–2005. (h) M. Ahmad, R. G. Bergstrom, M. J. Cashen, Y. Chiang, A. J. Kresge, Mc Clelland, M. F. Powell, J. Am. Chem. Soc., 101, 1979, 2669–2677. (i) A. E. Reed, P. von R. Schleyer, Inorg. Chem., 27, 1988, 3969–3987. (j) P. P. Graczyk, M. Mikolajczsk, J. Org. Chem., 61, 1996, 2995–3002.
- 29 V. Jagannadham, T. L. Amyes, J. P. Richard, J. Am. Chem. Soc., 115, 1993, 8465.
- 30 D. P. N. Satchell, R. S. Satchell, Chem. Soc. Rev., 19, 1990, 55–81.
- 31 J. K. Pau, M. B. Ruggera, J. K. Kim, M. C. Caserio, J. Am. Chem. Soc., 100, 1978, 4242–4248.
- 32 O. Exner: in N. B. Chapman, J. Shorter (eds): Advances in Linear Free Energy Relationships, Plenum Press, London, New York, p. 32 (1972).
- 33 Okuyama, T. in S. Oae (ed): Reviews on Heteroatom Chemistry, Vol. 1, MYU, Tokyo.
- 34 D. Ohlmann, C. M. Marchand, H. Grützmacher, G. S. Chen, D. Farmer, A. Glase Currao, R. Nesper, H. Pritzkow, Angewandte Chemie, 35, 1996, 300–303.
- 35 C. U. Pittmann, Jr., S. P. McManus, J. W. Larsen, Chem. Rev., 72, 1972, 357–438.
- 36(a) F. G. Bordwell, G. E. Drucker, N. H. Andersen, A. D. Denniston, J. Am. Chem. Soc., 108, 1986, 7310–7313. (b) F. G. Bordwell, J. E. Bares, J. E. Bartmess, G. E. Drucker, J. Gerhold, McCollum M. Van Der Puy, N. R. Vanier, W. S. Matthews, J. Org. Chem., 42, 1977, 326–332.
- 37 M. T. Jernigan, E. L. Eliel, J. Am. Chem. Soc., 117, 1995, 9638–9644.
- 38 J. E. Bartmess: Gas Phase Acidity Scale as of October, Vol. 25 (1994).
- 39 H. J. Reich, J. P. Borst, R. R. Dykstra, Tetrahedron, 50, 1994, 5869–5880.
- 40(a) A. G. Abatjoglou, E. L. Eliel, L. F. Kuyper, J. Am. Chem. Soc., 99, 1977, 8262–8269. (b) E. L. Eliel, A. A. Hartmann, A. G. Abatjoglou, J. Am. Chem. Soc., 96, 1974, 1807–1816. (c) O. Hofer, E. L. Eliel, J. Am. Chem. Soc., 95, 1973, 8045–8050. (d) E. L. Eliel, A. Abatjoglou, A. A. Hartmann, J. Am. Chem. Soc., 94, 1972, 478. (e) A. A. Hartmann, E. L. Eliel, J. Am. Chem. Soc., 93, 1971, 2572–2573.
- 41(a) L. Xie, A. Streitwieser, J. Org. Chem., 60, 1995, 1339–1346. (b) P. Speers, K. E. Laidig, A. Streitwieser, J. Am. Chem. Soc., 116, 1994, 9257–9261. (c) L. Xie, D. A. Bors, A. Streitwieser, J. Org. Chem., 57, 1992, 4986–4990.
- 42 P. von R. Schleyer, T. Clark, A. J. Kos, G. W. Spitznagel, C. Rohde, D. Arad, K. N. Houk, N. G. Rondan, J. Am. Chem. Soc., 106, 1984, 6467–6475.
- 43 W. T. Borden, E. R. Davidson, N. H. Andersen, A. D. Denniston, N. D. Epiotis, J. Am. Chem. Soc., 100, 1978, 1604–1605.
- 44 I.-M. Lehn, G. Wipff, J. Am. Chem. Soc., 98, 1976, 7498–7505.
- 45 F. Bernardi, A. Mangini, G. Tonachini, P. Vivarelli, J. Chem. Soc. Perkin Trans. II, 1985, 111–114.
- 46 K. B. Wiberg, H. Castejon, J. Am. Chem. Soc., 116, 1994, 10489–10497.
- 47(a) M. S. Alnajjar, X.-M. Zhang, J. A. Franz, F. G. Bordwell, J. Org. Chem., 60, 1995, 4976–4977. (b) F. G. Bordwell, X.-M. Zhang, J. Am. Chem. Soc., 116, 1994, 973–976. (c) F. G. Bordwell, X.-M. Zhang, M. S. Alnajjar, J. Am. Chem. Soc., 114, 1992, 7623–7629.
- 48 P. Arya, C. Samson, M. Lesage, D. Griller, J. Org. Chem., 55, 1990, 6248–6250.
- 49
G. Leroy,
M. Sana,
C. Wilante,
J. Mol. Struct. (Theochem.),
234,
1991,
303–328.
10.1016/0166-1280(91)89020-2 Google Scholar
- 50 J. L. Ginsburg, J. C. Baum, R. F. Langler, J. Phys. Chem., 94, 1990, 1750–1755.
- 51 C. Hansch, A. Leo, R. W. Taft, Chem. Rev., 91, 1991, 165.
- 52 C. D. Johnson: The Hammett Equation, Cambridge University Press, Cambridge (1973).
- 53 J. T. Edward, Chem. Ind. (London), 1955, 110L.
- 54 R. U. Lemieux, Pure Appl. Chem., 25, 1971, 527.
- 55(a) E. Juaristi, G. Cuevas, Tetrahedron, 48, 1992, 5019–5087. (b) See P. A. Kollman, et al, J. Org. Chem., 61, 1996, 3662–3668, for a very recent discussion.
- 56 W. F. Bailey, H. Common, E. L. Eliel, K. B. Wiberg, J. Am. Chem. Soc., 100, 1978, 2209.
- 57 M. Mikolajczyk, P. P. Graczyk, M. W. Wieczorek, J. Org. Chem., 59, 1994, 1672–.
- 58 G. Boche, Angewandt Chemie, I. Ed. Eng., 28, 1989, 277–297.
- 59 R. Amstutz, J. D. Dunitz, D. Seebach, Angewandt Chemie, 20, 1981, 465–466.
- 60(a) C. Gaze, B. C. Gilbert, J. Chem. Soc., Perkins Trans. II, 1979, 763–769. (b) A. L. J. Beckwith, S. Brumby, J. Chem. Soc., Perkin Trans. II, 1987, 1801–1807.
- 61 M. S. Alnajjar, X.-M. Zhang, J. A. Franz, F. G. Bordwell, J. Org. Chem., 60, 1995, 4977.
- 62(a) H. Lankamp, W. T. Nauta, C. MacLean, Tetrahedron Lett., 2, 1968, 249–254. (b) J. M. McBride, Tetrahedron, 30, 1974, 2009–2022.
- 63 Compare with T. Gade, M. Streek, J. Voss, Chem. Ber., 121, 1988, 2245.
- 64 J. Klaveness, K. Undheim, Acta Chim. Scand. B, 37, 1983, 687–690.
- 65 J. K. Grime (ed): Analytical Solution Calorimetry, John Wiley and Sons, New York (1985).
- 66 J. J. Gajewski, K. E. Gilbert, J. McKelvey: in D. L. (ed): Advances in Molecular Modeling, JAI Press, Greenwich, CT (1990).
- 67 A. L. J. Beckwith, A. A. Zavitsas, J. Am. Chem. Soc. 117, 1995, 607–614.
- 68 John A. Dean: Lange's Handbook of Chemistry, 14th Ed., McGraw-Hill, Inc., New York (199?).




