Evidence supporting a role for circulating macrophages in the regression of vascular remodeling following sub-chronic exposure to hemoglobin plus hypoxia
These authors contributed equally to this manuscript.
Abstract
Macrophages are a heterogeneous population with both pro- and anti-inflammatory functions play an essential role in maintaining tissue homeostasis, promoting inflammation under pathological conditions, and tissue repair after injury. In pulmonary hypertension, the M1 phenotype is more pro-inflammatory compared to the M2 phenotype, which is involved in tissue repair. The role of macrophages in the initiation and progression of pulmonary hypertension is well studied. However, their role in the regression of established pulmonary hypertension is not well known. Rats chronically exposed to hemoglobin (Hb) plus hypoxia (HX) share similarities to humans with pulmonary hypertension associated with hemolytic disease, including the presence of a unique macrophage phenotype surrounding distal vessels that are associated with vascular remodeling. These lung macrophages are characterized by high iron content, HO-1, ET-1, and IL-6, and are recruited from the circulation. Depletion of macrophages in this model prevents the development of pulmonary hypertension and vascular remodeling. In this study, we specifically investigate the regression of pulmonary hypertension over a four-week duration after rats were removed from Hb + HX exposure with and without gadolinium chloride administration. Withdrawal of Hb + HX reversed systolic pressures and right ventricular function after Hb + Hx exposure in four weeks. Our data show that depleting circulating monocytes/macrophages during reversal prevents complete recovery of right ventricular systolic pressure and vascular remodeling in this rat model of pulmonary hypertension at four weeks post exposure. The data presented offer a novel insight into the role of macrophages in the processes of pulmonary hypertension regression in a rodent model of Hb + Hx-driven disease.
Introduction
Pulmonary hypertension (PH) is a multi-factorial disease leading to progressive right heart failure and death.1 The primary pathophysiological parameter that defines PH is a mean pulmonary arterial (PA) pressure exceeding 25 mm Hg2 and PH occurs in a variety of clinical situations categorized into six groups by the World Health Organization (WHO).1, 2 The type of PH that manifests in patients with hemolytic anemias such as sickle cell disease (SCD) is classified to group 5, where PH is secondary to either pre- (52.6% of patients) or post-capillary driven disease (47.4% of patients) and is the primary cause of death. In SCD patients, pre- or post-capillary-driven PH processes are causally linked to the pulmonary vasculopathy caused by free hemoglobin (Hb) and its degradation products, heme, and iron, resulting in nitric oxide depletion hypoxia and macrophage reprogramming.3, 4 We have developed an animal model that combines chronic Hb infusion and hypoxia (HX) to mimic intravascular hemolysis and impaired tissue oxygenation to approximate PH in the SCD population.5-7
An increase in interstitial and perivascular macrophages is observed in rodent and human PH and is implicated in the pathogenesis and vascular remodeling. Macrophages also play a significant role in removing Hb and heme through haptoglobin and hemopexin trafficking and senescent red blood cell removal. However, excess macrophage iron can increase iron extrusion into the physiological environment.8 In our PH model, we have observed a macrophage phenotype characterized by intracellular accumulation of iron, robust expression of heme oxygenase 1 (HO-1), and mediators of hypoxia (HX)-induced PH such as endothelin-1 (ET-1) and interleukin 6 (IL-6). Furthermore, by depleting circulating macrophages with gadolinium trichloride (GdCl3), we observed a significant attenuation of the PH response, suggesting a critical role for macrophages in the development and progression of PH during exposures to Hb and HX.7
Macrophages are essential in the resolution of inflammation and tissue repair after injury. However, there is a dearth of studies addressing the role of macrophages in regression of established PH. The present study is designed to determine whether iron-laden macrophages persist after cessation of Hb and concomitant Hx exposure. Further, we sought to determine if macrophages have a role in reversing medial hypertrophy of pulmonary vasculature. While this study is an observational examination of PH regression in rats after Hb plus HX exposure, we also ask a critical prospective question. We hypothesized that a marked reduction in adventitial macrophages rich in iron, HO-1, IL-6, and ET-1 would occur within a month after cessation of Hb plus HX, and the elimination of circulating macrophages with Gadolinium Chloride (GdCl3) would slow the recovery process. This hypothesis is based on the insufficiently explored role of macrophages in the reversal of pulmonary vascular remodeling. Data described herein define the processes in a relevant animal model of group 5 PH.
Materials and methods
Animals
Male Sprague-Dawley rats were obtained from a commercial vendor (Jackson Laboratories, Bar Harbor, ME, USA). All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at our University. All animals survived the surgical procedure and indwelling catheterization into the jugular vein. None of the rats exhibited any signs or symptoms indicative of systemic infection. After surgery, wounds healed within 14 days.
Rats (n = 28) were randomly assigned to one of four groups: (1) normoxic control (NX; n = 6); (2) hypoxic control (HX; n = 6); (3) hemoglobin plus hypoxia (Hb + HX; n = 8); (4) hemoglobin plus hypoxia treated with gadolinium chloride (Hb + HX + GdCl3; n = 8).
iPrecio infusion pump placement
Programmable infusion pumps (iPRECIO, Tokyo, Japan) were placed subcutaneously as previously described.5 iPRECIO pumps were programmed to deliver Hb (35mg/day or 250 ug/uL) or saline at an infusion rate of 6 mL/h for 35 days. Pumps were refilled with fresh aliquots of Hb or saline every three to four days. At a dose of 35 mg/day, we show that total plasma heme concentrations were in the range of values similar to a mild to moderate chronic hemolytic state observed in SCD.5, 6
Endotoxin and catalase-free human adult hemoglobin (HbA; (Fe2+))
Purified human endotoxin-free Hb (LPS < 0.5 endotoxin units, EU) was prepared from outdated blood as previously described, with an additional step to remove catalase by chromatography.9 Approximately 1 g of Hb protein was loaded onto a GE Healthcare glass column (HR 16/50:1 ½″ × 26″) packed with SuperoseÔ 6 (Code No. 17-0489-01, Amersham Biosciences, Upsala, Sweden) and run at 4°C and a flow rate of 2 mL/h. Hb protein was collected and was followed by buffer switching to 0.1 NaCl to remove catalase from the column. Several runs were pooled and concentrated to 200 mg/mL using Centricon Plus–70 with 30 kDa cutoff membrane filters (Millipore, Billerica, MA, USA). The starting composition of Hb was 96.5 ± 1.3% Fe2+, 3.50 ± 0.23% Fe3+, and no measurable hemichrome. Endotoxin concentration was <0.5 EU/mL as determined by the limulus ambocyte lysate assay and failed to activate macrophages in culture.
Hypoxia exposure
Rats were continuously exposed to simulated high altitude (5500 m, 18,000 ft) in a rodent hypobaric chamber facility as previously described.5-7 Animals were exposed to this atmosphere continuously for 35 days. It has been previously demonstrated that chronic hypoxia can increase pulmonary arterial pressure, right ventricular hypertrophy, and pulmonary vascular remodeling.
Echocardiography
To assess approximate time frames of the progression and resolution of PH echocardiographic analysis was performed at two-week intervals using a high-resolution in vivo microimaging Vevo770 system (Visual-Sonics). Care was taken to follow the guidelines for measuring cardiac physiology in rats established by the American Physiological Society.10 For each analysis, rats were anesthetized via an isoflurane/O2 mixture with induction at 4% and maintenance between 1.5% and 3%, and body temperature was maintained at 37°C via a heated surgical pad. As described by Cavasin et al.,11 pulse-wave Doppler imaging of the pulmonary outflow was recorded in the parasternal short-axis view at the level of the aortic valve. Animals were then monitored during the recovery from anesthesia; upon full recovery, they were returned to their respective housing
Gadolinium chloride
Gadolinium chloride (GdCl3) (Cat # G7532; Sigma) was dissolved in sterile 0.9% NaCl and delivered intravenously in a 1 mL bolus. Rats were treated with gadolinium chloride every three days at a dose of 10 mg/kg for four weeks. We previously demonstrated by qRTPCR analysis that this dose effectively depletes macrophages by depleting CD163+ and CD68+ cells in the liver.7
Endpoint blood pressure measurements
Pulmonary blood pressures were measured with a 1.9F pressure-volume catheter placed in the main PA in an open-chest procedure. Correct placement of the catheter was confirmed by observing the normal rise in diastolic pressure as the catheter was moved into the PA. Systemic blood pressure was monitored with a catheter inserted in the femoral artery. Cardiac performance was assessed using an ADV500 pressure-volume system (Transonic/Scisense), and data acquired and analyzed with Labscribe2 (iWorx). For all measurements, animals were anesthetized with 5% and maintained at 2% isoflurane, and body temperature was maintained at 37°C.
Blood and organ collection
Serial blood draws were conducted at baseline (pump placement) and every three days post initiation of Hb and hypoxia exposure. Blood was placed in an EDTA-K + vacutainer and a crit tube to analyze plasma and hematocrit, respectively.
Animals were exsanguinated via a carotid artery catheter; blood was placed in a vacutainer with EDTA and centrifuged. The plasma was collected, snap-frozen in liquid nitrogen, and stored at –80°C until analysis; 300 μL of blood was also placed in a crit-tube and spun to measure hematocrits. The femoral artery was severed, and the lungs, kidneys, spleen, and liver were perfused with PBS (120mL) via the right ventricle to remove blood. The kidneys, spleen, and liver were cut in two sections: one piece was placed in 10% formalin and the other in AllProtect (Qiagen, Valencia, CA, USA). The right lung lobes were tied off at the right main bronchus, removed, and a piece was placed in AllProtect, and another portion was snap-frozen. The left lung was fixed with 10% formalin (∼3 mL) by airway inflation under constant pressure at 25 cm H2O pressure, and the entire lung-heart bloc was removed. The hearts were removed, and the right and left ventricles plus septum were isolated. The right ventricle (RV) and left ventricle with the septum (LV + S) were weighed for assessment of the Fulton Index (right ventricular hypertrophy; RV/LV + S). After 24 h, the formalin-fixed organs were removed from 10% formalin and placed in 70% ethanol, and prepared following standard methods for morphometric and immunohistochemical analyses.
Materials
The following antibodies were used: b actin (cat # ab8226), 1:1000 (Abcam); smooth muscle actin (cat #ab5694 and ab7817), 1:1000 (Abcam); heme oxygenase (HO1; cat # ab13248), 1:250 (Abcam); interleukin 6 (IL6; cat # ab9324), 1:1000 (Abcam); endothelin 1 (ET1; cat # ab117757), 1:500 (Abcam); CD68 (cat # ab955), 1:500 (Abcam); intracellular adhesion molecule 1 (ICAM; cat # ab2213), 1:250 (Abcam). Lung sections were incubated with a fluorescent secondary antibody either AlexaFluor 488; 1:1000 or AlexaFluor 555; 1:1000 (Invitrogen; Carslbad, CA, USA) or a biotinylated secondary horse anti mouse or horse anti-rabbit, 1:1000 (Vector Labs, Burlingame, CA, USA).
Western blot analysis
Lungs were homogenized using a TissueMiser (ThermoFisher, Waltham, MA, USA) in RIPA buffer containing protease inhibitors; protein concentrations were determined using a Pierce BCA Protein Assay. Briefly, Western blot analysis was performed using 30 mg of sample protein run under denaturing and reducing conditions on Biorad Criterion Tris-HCl 12.5% gels with a Biorad SDS-Page blot system. All proteins were normalized to b-actin. Statistical analysis was determined from the fold difference from Nx (normoxic control) on the same gel. Gels were imaged on an Alpha Innotech gel documentation system (Protein Simple, Santa Clara, CA, USA), and densitometric analyses were performed using ImageJ software (version 1.44o, National Institutes of Health, USA).
Immunohistochemistry and morphology
Formalin-fixed, paraffin-embedded lung, kidney, and spleen tissue was sectioned by our university. Five-micrometer sections were stained with hematoxylin and eosin by standard procedures to assess the accumulation of perivascular cells as well as vessel wall thickness. Lung sections were also stained for smooth muscle actin utilizing a biotinylated horse anti-mouse immunoglobulin followed by a 3,3′-diaminobenzidine (DAB) for development to quantify small arterial muscularization.
The entire tissue field on each slide was scanned utilizing a Leica AperioCS2 microscope. Analyses of the scanned images were performed using the Aperio eSlide manager software (Leica, Buffalo Grove, IL, USA). Positively stained smooth muscle vessels were counted and described as either fully or partially muscularized. Any area that fell outside of the lung boundary was subtracted from the total area, and the number of either fully or partially muscularized vessels was normalized to area. Distal pulmonary vessels (outside diameter of 10–50 mm) were assessed for degree of circumferential a-smooth muscle actin-positive staining, indicative of muscularization. Proximal vessels (outside diameter of 50–250 mm) were analyzed for medial wall thickness at four points around the vessel circumference and for lumen diameter along two axes. Wall thickness is expressed as the ratio of medial wall thickness to lumen radius.
Histological analysis of nonheme iron deposition in lung tissue was detected using Perl's staining with DAB counterstain as previously described.5-7 For quantification, a total of 20 images at 20× magnification were obtained from sections of all animals. Within each of the 20 images, the total number of Perl's positive cells was counted and divided by 20.
Statistical analysis
For all groups, the mean SEM is reported. Statistical comparisons for data measurements were completed with either one-way analysis of variance12 for NX, HX, Hb + HX, and Hb + HX + GdCl3 treatment (endpoint analysis) or two-way ANOVA for analysis between time (echocardiography analysis). Echocardiography data were also analyzed by a univariate repeated measures test using JMP (SAS Cary, NC, USA). All Post hoc analyses were completed with unpaired, two-sided Student t-test with a Bonferroni correction unless otherwise noted. Statistical analyses were performed using GraphPad Prism (La Jolla, CA, USA) with statistical significance set at p ≤ 0.05.
Results
Macrophage depletion slows the resolution of pulmonary vascular remodeling in a hemoglobin plus hypoxia rat model of pulmonary hypertension
Hypoxia-induced vascular remodeling reverses upon reoxygenation. We demonstrate that rats exposed to HX with continuous low-level Hb infusion for five weeks develop a more severe PH than with Hx alone.5-7 In this study, we wanted to assess whether our PH model would be reversible after the withdrawal of Hb and HX and if macrophages have a role in this process. To this end, circulating monocytes/macrophages were depleted with GdCl3 during a four-week recovery period after cessation of both Hb and HX (Fig. 1a). Groups consisted of normoxic control (NX), hypoxic control (HX5wk), (Hb + HX5wk), and HX 5wk followed by four weeks normoxia (HX5wk; NX+4wk), Hb + HX 5wk followed by four weeks no Hb infusion in normoxia (Hb + HX5wk; -Hb + HX4wk) ± GdCl3 (Fig. 1b). Serial echocardiography and endpoint hemodynamics, Fulton index (RV/LV + S), and lung morphology/histology were used to assess the impact of macrophage depletion on PH resolution. The purpose of echocardiography was to understand approximate time frames of the progression and resolution of PH, and not as a primary indicator to identify exact differences among groups more suitable for endpoint analysis.
Echocardiography analysis revealed that Hb + HX or HX exposed rats demonstrated increased pulmonary artery acceleration time (PAAT) and RV wall thickness compared to NX cohorts at five weeks. Combining all Hb + HX rats at five weeks before GdCl3 treatment revealed lower PAAT in Hb plus HX rats than rats only exposed to Hx, reflecting higher PA pressures (Fig. 1b, inset) and more progressive PH (Fig. 1b–d). This main effect of Hb + HX is congruent with our previous studies demonstrating higher pulmonary arterial pressures in Hb plus HX rats vs. HX alone.5, 6 Analysis of serial echocardiography data shows a significant improvement of PAAT and RV wall thickness during the four-week recovery period (Fig. 1b–d). However, rats maintained on continuous Hb infusion plus HX had lower PAAT and greater RV wall thickness at two- and four-week time points of recovery (Fig. 1b and c) than HX alone rats (Fig. 1b–d).
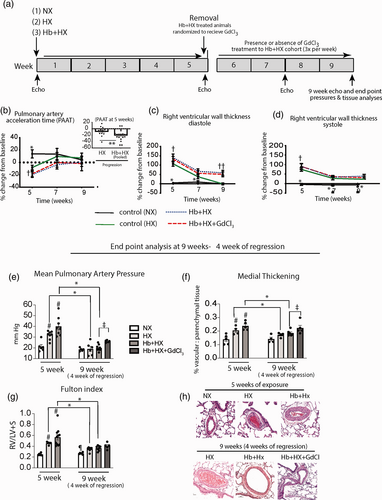
Cardiopulmonary analysis of the progression and the macrophage contribution in the resolution of hemoglobin (Hb) plus hypoxia (HX) mediated pulmonary hypertension. (a) Schematic of study design. Five weeks of exposure to Hb plus Hx followed by a four-week observational period after removal of Hb plus HX. (b–d) Echocardiography measurements of the pulmonary artery acceleration time and right ventricular wall thickness in diastole and systole at five weeks after progressive PH, and at two and four weeks after removal PH stimulus. (e–g) End point measurements of mean pulmonary artery pressure, pulmonary artery medial thickening, and right ventricular hypertrophy, and in a subset of rats sacrificed at five weeks and rats sacrificed after four weeks of recovery. (h) Microscopy visualization of H&E stained lung tissue in rats after five weeks of progressive PH (HX and Hb plus HX exposure) and after four weeks of recover. Normoxic rats were sacrificed at five weeks (n = 3) and nine weeks (n = 3). †p < 0.05 - five-week time point vs. seven- and nine-week time point; ††p < 0.05 Hb + HX and Hb + HX + GdCl3 vs. HX and NX cohorts at nine-week time point; #p < 0.05 vs. NX at five weeks; *p < 0.05- five-week vs. nine-week time points; ǂp < 0.05 Hb + HX vs. Hb + HX+GdCl3 at nine-week time point; ¶ p < 0.05 NX vs. all other groups at nine-week time point.
Invasive hemodynamic measurements obtained after four weeks of recovery show the elimination of circulating macrophages with GdCl3 prevented the complete resolution of mean pulmonary artery pressure (PAP) and pulmonary vascular remodeling as compared between the Hb + HX and Hb + HX + GdCl3 groups (Fig. 1e–h). These data suggest that a subset of circulating monocytes/macrophages recruited to the lung may be required for the complete regression of Hb + HX-induced vascular remodeling. While the Fulton index calculation (RV/LV + S) demonstrated RV hypertrophy was not completely resolved in any group (Fig. 1g), the comparisons between Hb + HX and Hb + HX + GdCl3 did not reach statistical significance. However, by comparing HX + GdCl3 treated animals compared to HX alone, we noted greater mean PAP, medial thickening, and Fulton index (Supplemental Data Fig. 1). When combining all the treated and non-treated GdCl3 animals into two groups, data suggest eliminating circulating macrophages with GdCl3 slows the resolution of PAP, and medial thickening, RV hypertrophy, at four weeks (Supplemental Fig. 1). While other factors are at play in the reversal of PH, taken together these data suggest the circulating macrophage may work in concert with other cell types to either hasten the reversal process or are needed for complete resolution.
Gadolinium decreases cd163 positive but increases cd68 positive macrophages in the lung after four weeks of reoxygenation
We demonstrate iron accumulation in the lung adventitial macrophages by five weeks of chronic Hb exposure with concomitant HX, and depletion of circulating macrophages prevented PH development in this model.5-7 Initially, we sought to determine whether macrophages containing iron persist at four weeks after cessation of the stimulus. Tissue Perls-DAB staining of the lung revealed that some iron accumulation persists in the adventitia and parenchymal cells in the Hb + HX rats (Fig. 2a). However, we cannot conclude if the early iron-containing macrophages survive in the lung for extended periods. As expected, we observed virtually no iron in the NX control animals and minimal iron in the HX and Hb + HX + GdCl3 cohorts (Fig. 2a). Macrophage activation and polarization to M2 phenotype promote PH and vascular remodeling. We characterized the macrophages in the lungs by staining for cd68, a pan macrophage marker, and cd163, a marker for the M2 phenotype. Levels of cd68, a pan macrophage marker, were lower in HX and Hb + HX recovery groups but was significantly higher in Hb + HX + GdCl3 treated recovery group (Fig. 2b and c). Staining for cd163, a marker for M2 macrophage shows a significant decrease in the Hb + HX and Hb + HX + GdCl3 recovery group but was not affected in the HX group compared to the HX group five-week time point (Fig. 2d–e). These data demonstrate that during regression, there is a decrease in the M2 macrophage phenotype. Interestingly there is also an increase in a subset of macrophages that appear resistant to GdCl3 treatment in the Hb + HX group (Fig. 2b–c).
Decreased IL-6 and ET1 expression in rat lungs during recovery
In rats exposed to Hb + HX for five weeks, there is increased iron accumulation in adventitial macrophages that express mediators of hypoxia-driven PH, IL-6, and ET-1.7 Here, we examined if IL-6 and ET-1 expression persisted in the residual iron overloaded macrophages after reoxygenation. Western blot analysis of lung lysates revealed decreased IL-6 in all groups of the recovery cohort. Furthermore, we observed IL-6 positive stain was predominantly in the media of distal pulmonary arteries of Hb + HX treated rats during regression and not in the iron-laden macrophages (Fig. 3a–c).
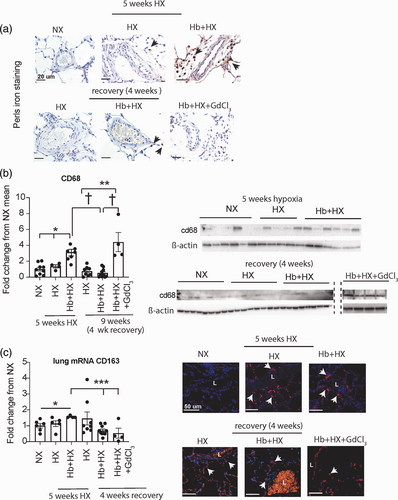
Lung iron accumulation and presence of cd68 and cd163 cell populations after five weeks of progressive PH and following four weeks of recovery. (a) Perls lung iron accumulation original magnification 60× Brown- iron, Black arrows- iron loaded cells (macrophages); (b) Western blot analysis of whole lung cd68; (c) mRNA quantification of cd163 in whole lung tissue; (d) Immunohistochemistry microscopy visualization for cells expressing cd163 in lung tissue sections of rats. Original magnification 40×. Red- cells expressing cd163. White arrows- positive cells.
In contrast to IL-6, we observed robust ET-1+ cells in Hb + HX rats located in both the adventitial and vascular compartments in distal pulmonary arteries (Fig. 3d–f). These areas were generally in the same locations as we observed and Iron+ cells, suggesting ET-1 persistence may be associated with iron-loaded macrophages (Fig. 3f). ET-1 was decreased in the Hb + HX + GdCl3 group, suggesting macrophages may be the dominant source of ET-1 in the lung (Fig. 3d). As expected, we observed minimal expression of Il-6+ and ET-1+ cells in both NX control rats and rats one month after chronic HX exposure. These results suggest that circulating macrophages increase ET-1 levels, but IL-6 levels were driven by hypoxia. Overall, these data demonstrate that the circulating macrophage influences PH mediators differentially during regression.
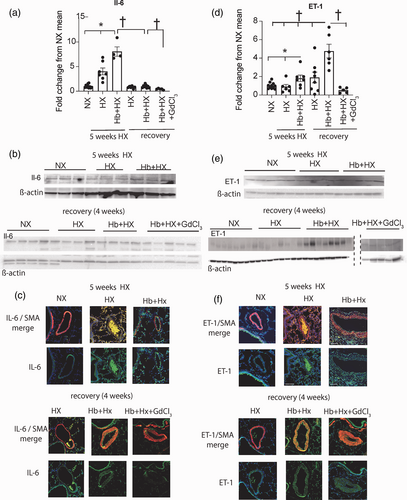
Quantification and location of lung IL-6 and ET-1: (a) Western blot quantification of IL-6; (b) representative western blot of IL-6; (c) Immunohistochemistry visualization of location of lung and IL-6. Original magnification 40×. (d) Western blot quantification of ET-1; (e) representative western blot of ET-1; (f) Immunohistochemistry visualization of location of lung and ET-1. Original magnification 20× Immunohistochemistry visualization of location of lung HO-1 and ET-1. Original magnification 40×. †p < 0.05 vs. Hb + HX at four weeks of regression; *p < 0.05 vs. HB+HX at five weeks of progressive PH; **NX vs. Hb + HX by Students t test.
Increased HO-1 expression during recovery
Expression of HO-1 is considered protective in PH and other vascular diseases. We observed the persistence of HO-1 in iron positive cells in the vessel wall, in the Hb + HX group like the macrophage expressing the Hb clearance phenotype in our previous studies.5-7 Interestingly, eliminating circulating macrophages during regression resulted in increased expression of HO-1+ in cells throughout the lung (Fig. 4a–c). HO-1+ cells are distributed throughout the vessel wall and highly expressed in the media of distal pulmonary arteries of the Hb + HX + GdCl3 rats (Fig. 4a–c), demonstrating a shift in lung HO-1 cellular expression with depletion of circulating macrophages. Congruent with immunofluorescence staining, Western blot analysis of whole lung tissue confirmed that HO-1 expression was significantly greater in the Hb + HX + GdCl3 treated rats (Fig. 4c). These results differ from those observed in the preventive study with GdCl3 treatment, where HO-1 expression did not increase in the vessel wall. In the NX and HX cohorts, we noted HO-1 expression was low in parenchymal and adventitial cells of the lung (Fig. 4a–c). Within the five-week main study, Hb + HX caused diffuse fibrosis in peripheral pulmonary vessels consistent with muscularized remodeled lung vasculature (Fig. 4d). Fibrosis was not nearly as extensive in NX or the HX groups. During the four-week regression phase, Hb + HX + GdCl3 treated rats demonstrated diffuse interstitial and muscularized tissue fibrosis (Fig. 4d), suggesting that depletion of peripheral macrophages was not a factor in the progression of fibrosis during the four-week regression phase.
Discussion
Studies have shown that vascular remodeling reverses spontaneously upon reoxygenation in hypoxic PH.13, 14 Our Hb + HX treatment model of PH shows a gradual reversal of PH in rats after withdrawal of Hb + HX as measured at four weeks by a decrease in mean pulmonary artery pressures, RV hypertrophy, and medial thickening of arteries. Although additive in the initial five weeks of exposure, our data demonstrate cessation of Hb plus HX resolves as quickly as PH driven by HX alone5-7 and also does not follow a fatal and unresolvable PH trajectory such as that seen in Sugen plus hypoxia PH rat model.15-17
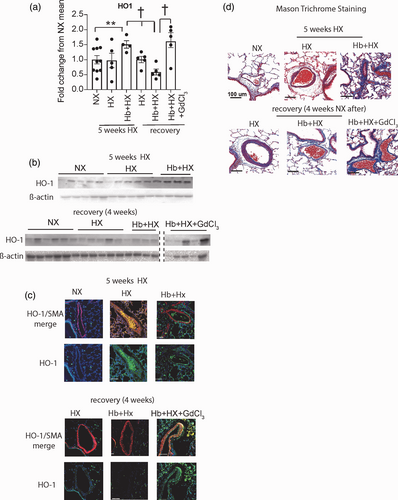
Quantification and location of lung heme oxygenase-1 (HO-1) and Masons Trichrome staining. (a) Western blot quantification of HO-1; (b) Representative western blot of HO-1; (c) Immunohistochemistry visualization of location of lung and HO-1. Original magnification 40×; (d) Masons trichrome staining; Blue- collagen. Original magnification 20×. †p < 0.05 vs. Hb + HX at four weeks of regression; **NX vs. Hb + HX by Students t test.
Macrophages are a heterogeneous population, and two major phenotypes (M1 and M2) with opposing inflammatory and anti-inflammatory functions have been described in the lung.18 Studies suggest that macrophages isolated from PAH patients exhibit the M2 phenotype and are responsible for promoting smooth muscle proliferation and migration.19 An increase in M2/M1 ratio is thought to promote vascular remodeling.19 In our study cd163, mRNA levels were lower in both the Hb + HX groups in the presence and absence of GdCl3. In mice with cd68 genetic deletion study, a decrease in macrophages in male mice altered the M1/M2 ratio, and increased basal RV pressures were observed.19 In our study, cessation of Hb + HX reduced the number of cd163 positive cells. However, we found a significant increase in cd68 positive macrophages in the GdCl3 treated cohort, showing increased pulmonary arterial pressures (Fig. 1). Our data cannot assess whether mean PAP increases are due to a skew in M2/M1 ratio. The cd68 positive macrophages may be from a resident population since GdCl3 decreases circulating monocyte-macrophage populations. There is evidence that alveolar macrophages can divide and self-renew without being replaced by stable circulating monocytes.20 Our group also has reported that the activation and expansion of alveolar and interstitial macrophages occur from different compartments during vascular remodeling at different time points in hypoxic PH.21 Whether macrophages from different compartments contribute to the resolution phase requires a detailed characterization of the subsets of macrophages at different time points during the regression phase.
We observed that the deletion of circulating macrophages with GdCl3 in the recovery phase prevented the complete reversal of pulmonary arterial pressures and vascular remodeling, indicating that macrophages facilitate PH resolution (Fig. 1a–h; Supplemental Fig. 1). Macrophages, by increasing inflammation, contribute to PH progression.22 Numerous studies have shown that deleting macrophages in different preclinical models by chemical or genetic means prevents vascular remodeling and PH development.21, 23 In our reversal study, the pro-inflammatory cytokine IL-6 returned to baseline levels upon withdrawal of Hb + HX, and the deletion of macrophages had no additional effect. However, ET-1 levels were high in the Hb/HX group even after withdrawal of Hb/HX, which was eliminated by GdCl3 treatment, suggesting that ET-1 was secreted by circulating macrophages. More recently, a study showed that macrophage-derived PDGF is a driver of PASMC growth and vascular remodeling.24 Therefore, in addition to pro-inflammatory markers macrophages may be the source of peptides that increase vascular constriction and remodeling.
Paradoxically, even with reduced IL-6 and ET-1, in the GdCl3 treated group, mean PAP and vascular remodeling were higher than NX mice. Other studies also show that macrophage depletion attenuates vascular remodeling and does not affect hemodynamics.25 The inhibition of Cx3cr1 in monocrotaline and hypoxia models of PH attenuated macrophage infiltration to the lung, preventing vascular remodeling but not improving hemodynamics.25, 26 In cd68 KO mice, elevated pressures are observed in a normoxic environment suggesting a subset of macrophages are required to maintain homeostasis in the vascular system.19 Although in the GdCl3 treated group, we had increased levels of cd68 positive cells, whether they contribute to pressure cannot be concluded from our data. However, increased HO-1 expression in smooth muscle cells increases blood pressure by modulation of the NO pathway.27, 28 We observed a significant increase in HO-1 in the vessel wall of rats treated with GdCl3 (Fig. 4). Whether this contributes to the residual pressures will need further studies. This contrasts with other studies using transgenic mice where HO-1 is protective in PH.29 HO-1 levels have shown to be necessary for the beneficial effects of simvastatin in regression of established PH.30
The heterogeneous population of myeloid cells in the lungs is essential for maintaining homeostasis and regulating inflammation, injury, and remodeling.31 Therefore, microenvironments may influence macrophage phenotype, thereby explaining many opposing macrophage functions. Evaluation of our data suggests that a subset of pro-PH macrophages is likely being recruited out of the lung and replaced by recruitment of other circulating macrophage phenotypes programmed to reverse the remodeling process. The current study's limitation is that we did not characterize a unique subset of macrophages expressing an apparent pro-resolution phenotype that reverses medial hypertrophy. We have not yet used advanced techniques like flow cytometry to identify the populations of macrophages involved in the resolution. Advanced lineage tracing and single-cell transcriptomics will help determine the different macrophage populations in the progression and regression of PH. Follow-on studies will need to include metabolomics/proteomic analysis on circulating and lung macrophages to characterize further the pro-resolution macrophage phenotype, an area we are actively working on. This knowledge could help develop novel therapies targeting monocyte/macrophages to trigger programming to counteract PH rather than aid the disease process.
The persistence of pro-PH macrophages after cessation of Hb and HX has implications in PH associated with hemolytic diseases such as human SCD. During 'crisis,' in patients with SCD, the pulmonary vasculature is exposed to increased Hb levels and its degradation products (heme and iron) and tissue hypoxia. We propose that repeated cycles of these events cause accumulation of the pro-PH macrophages and unresolved inflammation, a key component of PH in the SCD population.
The data in the current study confirm previous observations that there is general plasticity of the pulmonary vasculature.15, 32, 33 In animal models, hypoxia-induced vascular remodeling can be reversed.16, 17, 34 Whether vascular remodeling can be reversed in human PH is not known. There are cases that severe PH has been resolved once the root cause was identified, isolated, and treated.35 Nevertheless, investigating how PH resolves in animal models may provide important insights into this critically important question.
Conclusion
In summary, supporting our original hypothesis we show that the circulating monocyte/macrophage population contributes to the resolution of unstable pulmonary hemodynamics and protein expression in rats after four weeks of recovery from active Hb plus hypoxia-mediated PH (five weeks of exposure to Hb plus hypoxia). While other factors are at play in the reversal of PH, taken together these data suggest the circulating macrophage may work in concert with other cell types to either hasten the reversal process or needed for complete resolution. Further, our data show that despite resolution of medial hypertrophy, a subset of macrophages expressing a pro-PH phenotype characterized by high iron content and ET-1 expression persists in the pulmonary adventitia of distal pulmonary arteries. These data highlight the agility of the innate immune system responding during and after noxious stress on the pulmonary vasculature and may lead to therapies targeting the macrophage to aid in reversing PH.
Author contributions
Designing research studies: VK, D Swindle, DIP, D Strassheim, MAF, ED, KRS, KH, RN, PWB, and DCI; Conducting experiments: VK, D Swindle, DIP, PWB, and DCI; Acquiring data: VK, D Swindle, DIP, PWB, and DCI; Analyzing data: VK, D Swindle, DIP, D Strassheim, MAF, ED, KRS, KH, RN, PWB, and DCI; Preparing figures: VK, DIP, PWB, and DIP; Writing manuscript: VK, D Swindle, DIP, D Strassheim, MAF, ED, KRS, KH, RN, PWB, and DCI.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work is supported by the U.S. Department of Health and Human Services: 5P01HL014985-40, P0HL 152961, RO1HL 156526, R011HL 1598662, R01HL 161004.
Guarantor
DCI.
ORCID iDs
Kurt R Stenmark https://orcid.org/0000-0001-6918-6411
Paul W. Buehler https://orcid.org/0000-0003-0687-3008
David C. Irwin https://orcid.org/0000-0002-1743-8266
Supplemental material
Supplemental material for this article is available online.




