Whole-Genome Sequencing Reveals the Evolutionary Characterization of Bovine Coronavirus
Abstract
Bovine coronavirus (BCoV) is endemic worldwide, causing gastrointestinal and respiratory diseases in cattle, making it a topic of significant interest. The present study investigated the prevalence and evolutionary characterization of BCoV identified in Yunnan, China. The results indicated that the overall seroprevalence was 21.75% (65/285), with a higher rate (25.56%) in diarrhea samples than in healthy samples (15.24%). Meanwhile, PCR was used to detect BCoV pathogens in 616 fecal samples. The overall BCoVs positive rate in Yunnan was 28.73% (177/616), with diarrhea samples showing a higher rate (37.46%) compared to healthy samples (20.50%). The whole genomes of three BCoV strain were successfully amplified, namely, BCoV/YN1LC/2023, BCoV/YNZT/2023, and BCoV/YNLP/2023. These identified strains showed high homology to strains derived from calf diarrhea and respiratory samples than to the classical and vaccine strains. Sequence analyses revealed that seven consistent amino acid mutations in the S protein of three BCoV identified strains, including M11/T, K115/D, N146/I, D148/G, L154/F, N499/S, and N509/H. Additionally, mutations L5/P, N49/T, and L392/I were observed in the HE protein, and mutations L53/Q, M386/T, M387/I, S423/I, and Y441/F were found in the N protein. Identified as a recombinant strain, the BCoV/YNLP/2023 displays a unique mutation S501/F in the S protein, D66/G in the HE protein, and the 206SRA208 deletion in the N protein. Phylogenetic analysis suggested that the identified strains could be the predominant strains in Yunnan Province or even in Southwest China, demonstrating geographic clustering. These data highlight BCoV’s high prevalence and evolutionary characterization in Yunnan, China, providing valuable information for the effective prevention and control of BCoV in the future.
1. Introduction
Bovine coronavirus (BCoV) causes intestinal and respiratory diseases in cattle, significantly impacting the growth of the cattle industry. BCoV are enveloped, nonsegmented, and positive-sense RNA viruses classified within the order Nidovirales, family Coronaviridae, subfamily Orthocoronavirinae, genus Betacoronavirus, and subgenus Embecovirus [1]. BCoV is implicated in the pathogenesis of three distinct clinical syndromes: enteric disease in neonatal calves, winter dysentery in adult cattle, and respiratory tract infections affecting cattle of all age groups [2]. Enteric BCoV (EBCV) infection impacts both the small and large intestines, leading to the destruction of the villi and resulting in severe diarrhea [3]. The occurrence of winter dysentery in adult dairy cattle can lead to a sharp decline in milk production, resulting in substantial financial losses [4]. Since the first detection of BCoV in 1972, positive cases caused by BCoV infection have been reported globally, in areas such as in the Americas, Europe, Asia, Oceania, and even Africa [5–11]. BCoV infection epidemics have been reported in Iran, China, India, Thailand, Vietnam, and other countries across Asia [12].
BCoV has a genome of approximately 31 kb, which encodes five main structural proteins: the spike (S), the transmembrane (M), the nucleocapsid (N), the small membrane (E), and the hemagglutinin/esterase (HE) [13]. The coronavirus (CoV) S protein plays a crucial role in the viral invasion of host cells and determines viral tropism, pathogenicity, and host range. The S protein consists of two subunits, S1 and S2. The S1 subunit is the spherical part of the S protein that binds to the cellular receptor, while the S2 subunit is the stem part of the S protein that mediates the membrane fusion process [14–16]. The sequence of the S glycoprotein is highly variable and mutations in this region are associated with changes in antigenicity and viral pathogenicity, making it important for genotyping viruses [17]. The HE protein exhibits an esterase receptor-destroying function and it could play a crucial role in viral entry, acting as a secondary viral attachment protein to initiate the infection [18].
BCoV shares common ancestors with human coronavirus OC43 (HCoV-OC43) and porcine hemagglutinating encephalomyelitis virus (PHEV) that are relatively recent [19]. HECV-4408, a strain of human enteric CoV, was initially found in a child’s stool with diarrhea and showed a closer genetic and antigenic relationship to BCoV than to HCoV-OC43 [20]. Besides infecting cattle, BCoV can also be transmitted to humans, domesticated and wild ruminants, including sheep, goat, yak, sambar, deer, and camel. This suggests potential cross-species transmission of the virus [21]. China, a major animal husbandry nation, faces new challenges in preventing and controlling bovine diseases due to the increasing number of beef cattle and dairy cattle being bred and introduced. BCoV negatively impacts the cattle industry, causing reduced milk production, loss of body condition, and significant economic losses [12, 22]. Currently, BCoV is considered widespread in China in both its enteric and respiratory forms, including BCoV infections in Yunnan Province. However, comprehensive investigations on the prevalence and genetic evolution of BCoV in Yunnan Province remain scarce. Therefore, serological and molecular epidemiological investigations to understand the prevalence and genetic characteristics of BCoV in Yunnan Province, China, can provide essential data and a theoretical basis for mapping BCoV prevalence and formulating effective prevention and control strategies.
To investigate the prevalence and evolution of BCoV in Yunnan Province in China, the study conducted serological and pathogen detection and genetic evolutionary analysis. Our aim is to provide novel epidemiologic information about BCoV in Southwest China. This research would provide invaluable insights to help develop effective prevention and control strategies against BCoV infection.
2. Materials and Methods
2.1. Sampling
During the period of 2021–2023, we collected 285 blood samples from five different breeds of either diarrheic or healthy cattle from seven cities or prefectures in Yunnan Province: Kunming (N = 26), Qujing (N = 15), Zhaotong (N = 91), Chuxiong (N = 47), Honghe (N = 59), Lincang (N = 17), and Baoshan (N = 30). The 285 blood samples consisted of 26 from shorthorn cattle, 47 from Dianzhong cattle, 138 from Hereford cattle, 59 from Angus cattle, and 15 from Simmental cattle. A total of 616 fecal samples were gathered from 16 different breeds of either diarrheic or healthy cattle in 11 cities or prefectures in Yunnan Province: Kunming (N = 22), Qujing (N = 19), Zhaotong (N = 64), Chuxiong (N = 16), Honghe (N = 91), Wenshan (N = 11), Lincang (N = 272), Baoshan (N = 52), Diqing (N = 23), Dehong (N = 28), and Pu’er (N = 18). Out of the 616 fecal samples collected, there were 49 from Red River yellow cattle, 6 from Diandongnan buffalo, 18 from Jiangcheng yellow cattle, 7 from Wenshan cattle, 4 from Murrah buffalo, 9 from Yunnan humped cattle, 13 from Mithun cattle, 6 from Dehong buffalo, 23 from Diqing yellow cattle, 16 from Dianzhong cattle, 22 from Shorthorn cattle, 50 from Angus cattle, 310 from Hereford cattle, 46 from Holstein cattle, 8 from Jersey cattle, and 29 from Simmental cattle. None of the cattle had been vaccinated against BCoV. Blood was drawn from the jugular vein using a collection tube, with a volume of ≥3 mL. The serum was then separated through centrifugation at 3000 rpm for 10 min and stored in −80°C. For fecal sample collection, a self-sealing bag was used to grab a small rub of cow feces or a sterile cotton swab was employed to smear deep within the anus. The sample was then placed into a 5 mL Eppendorf tube containing 0.9% saline and transported to the laboratory packed in ice. Once in the lab, 0.5 g fecal samples were mixed with 1.5 mL saline and kept at 4°C overnight. The mixture was then divided into 2 mL centrifuge tubes and centrifuged at 8000 rpm for 10 min. The supernatant was collected, filtered by a 0.22 µM filter, and stored at a −80°C.
2.2. Antibody and Pathogen Detection of BCoV
We performed BCoV IgG antibody screening on 285 processed serum samples from seven regions, following the instructions of the BCoV Antibody Detection Kit (Shuhua Biotechnology Co. Ltd, Shanghai, China). Viral RNA was extracted from supernatants using the RNAiso Plus RNA Kit (Takara, Shiga, Japan) according to the manufacturer’s instructions. The cDNA of BCoV was synthesized using the TiScriptTM cDNA Synthesis Kit (Bio Rad, Hercules, CA, USA) as per the manufacturer’s instructions. The detection of the BCoV pathogen was performed using the RT-qPCR method for the S gene developed in this study. The primer sequences used were: F 5′-CGACTTTTGCTGTTATAGGA-3′ and R 5′-TGTAGAACCTGAAGTAGGG-3′. The amplification reactions with the primers were conducted in a 20 μL reaction volume containing 0.4 μM forward primer, 0.4 μM reverse primer, 1.5 μL of genomic DNA, 10 μL of SsoFast EvaGreen Supermix (Bio Rad, Hercules, CA, USA), and an appropriate volume of diethylpyrocarbonate (DEPC) H2O. The cycling parameters were set at 39 cycles of 95°C for 15 s, 51–56°C for 20 s and 65°C for 5 s, followed by 95°C for 5 min, using the Bio Rad CFX 96 Real Time detection system (Bio Rad, Hercules, CA, USA).
2.3. Complete Genome Sequencing of BCoV Strains
In this study, we designed 16 pairs of primers for segmental amplification of BCoV’s entire genomes. These primers were synthesized by Kunming Bioengineering Co. Ltd. The primer sequences for the main genes are shown in Table 1. Genome cloning and Sanger sequencing were then performed.
| Primer name | Primer sequence (5′-3′) | Length of amplicons (bp) |
|---|---|---|
| 5UTR-F | GCGATATGCGTGAGTGCATCG | 725 |
| 5UTR-R | CTAAGCGTATAACCTAACCC | |
| OF1-F | GGCTTGGGTTATACCCTTAG | 2740 |
| OF1-R | ACCTTCCAAAACAGATCGCAACCAG | |
| OF2-F | TGTATGGAGACATCTGATTC | 2653 |
| OF2-R | AATACTTACGTCCGTCAC | |
| OF3-F | GTGACGGACGTAAGTATT | 2533 |
| OF3-R | GAGACATCAGCCATCTGC | |
| OF4-F | TCCTGTATATGGTCTGTGG | 2489 |
| OF4-R | CCATAGTCTGACGTCCTTG | |
| OF5-F | AGCGTCTCATGAATGGTT | 2741 |
| OF5-R | GCTGATAACGGCAGCAAT | |
| OF6-F | ATGCTCGTCTCGTACCCT | 2449 |
| OF6-R | CGACTAGGATCAGGATATGG | |
| OF7-F | AAGATGGATGTGGACGAT | 2678 |
| OF7-R | ATTGTTCATCAGGAGGAG | |
| OF8-F | GTTCTGGAAGCCACTGGTT | 2581 |
| OF8-R | AACGCGGTGCTACTCCTTT | |
| OF9-F | GGCTGTATGATGATTGTTGC | 1926 |
| OF9-R | TGACCTTCGGCTGTGTAAT | |
| HE-F | CCTACCAATGATGTTTCGCA | 1208 |
| HE-R | AGCCTAGTAGCATTATCCAC | |
| S1-F | GCATACTCAGTGGATTGT | 2558 |
| S1-R | CCACACAGTAACCACTACCT | |
| S2-F | ACTCTTGCGAACCAGCATTG | 1956 |
| S2-R | TCATCACTACAACCACCACA | |
| EM-F | TGTGGTCGTTGTTGTGATG | 1924 |
| EM-F | GAGAACCTAGAATAGTAGGG | |
| N-F | CCCTACTATTCATGGTTCTCTGG | 1112 |
| N-R | ATGTCGTCTGCAGTCAACTCTCTAA | |
| 3UTR-F | TCGACAGTTCCCCATTCTTG | 716 |
| 3UTR-R | GTGATTCATCCAATTGGCC |
2.4. Sequence Analysis of BCoV Strain
The three BCoV raw sequences obtained through amplification were compared using the Megalign software in the DNAStar 6.0 (DNAStar Inc., WI) software package. We selected BCoV complete genome, S, N, and HE protein sequences from the NCBI database as our reference sequences and identified strains in this study for homology comparison. Reference sequences for homology comparison included classical BCoV strains, vaccine strains, strains derived from diarrhea in calves, strains derived from winter dysentery in adult cattle, and strains derived from cattle carrying respiratory symptoms. A divergence analysis of the S, HE, and N protein of BCoV was conducted using WebLogo (http://weblogo.threeplusone.com/), an online software for creating sequence logo [23].
2.5. Phylogenetic Analysis of BCoV
The BCoV complete genome and S gene and reference sequences from GenBank were aligned using the Clustal W method in the MEGA 6 software [24]. The aligned sequences were subsequently used in MEGA 6 software to calculate the average genetic distance. Phylogenetic trees were constructed utilizing the neighbor-joining (NJ) and maximum likelihood (ML) methods. The confidence of each branch of the phylogenetic tree was tested for bootstrap using the resampling method (Bootstrap) with the number of tests set to 1000. Constituent tree files in nwk format were generated, and the ITOL online website (https://itol.embl.de) was used to enhance the visual presentation of the evolutionary tree [25].
2.6. Recombination Analysis of BCoV
The three BCoV Yunnan epidemic strains obtained in this study were tested for possible recombination events using seven methods: RDP, GENECONV, BootScan, MaxChi, Chimaera, SiScan, and 3Seq in the RDP4 (4.70) software. An event was deemed a recombination event if at least five of these seven algorithms identified the gene as undergoing recombination. To confirm the presence of gene recombination and breakpoints, recombination events were further verified using BootScan in SimPlot software (version 3.5.1). The parameters were set to a window size of 400 bp and a step size of 40 bp. This rigorous analysis ensures the accurate detection of recombination events, which are crucial in understanding virus evolution and its implications for disease control strategies.
2.7. Selection Pressure Analysis of BCoV S Gene
The Datamonkey online website (http://www.datamonkey.org/) was applied to analyze the S genes of the three BCoV Yunnan epidemic strains obtained in this study and 152 reference sequences from GenBank under selection pressure. This was done through the use of the single-likelihood ancestor counting (SLAC), mixed effects model of evolution (MEME), and fast unconstrained Bayesian approximation (FUBAR) methods. These allowed us to calculate the ratio between nonsynonymous (dN) and synonymous substitutions (dS) at the average number of nucleotide substitution sites, as well as infer the rates of dN and dS substitutions using the fixed effects likelihood (FEL) approach. This analysis helps clarify the selection pressure acting on the S gene. This analysis also included an exploration of evolutionary pressures of the Yunnan strains, providing further insights into the potential immune response elicited by these strains.
2.8. Estimation of Differentiation Time and Evolutionary Rate Based on BCoV S Gene
In this study, we used the BioAider software to align the S genes of the three BCoV Yunnan identified strains with 135 reference strains. The best fitting model for analyzing the BCoV S gene in this study using PhyloSuite software was determined to be the GTR + F + G4 model. BEAST input files were generated using BEAUti, while nucleotide substitution rates and the time to the most recent common ancestor (tMRCA) for each locus/year were estimated using the Bayesian Monte Carlo Markov chain (MCMC) method. We checked for convergence and mixing using Tracer software (v1.7). We then generated maximum clade credibility (MCC) trees with TreeAnnotator 1.7.4 software and visualized the tree using Figtree 1.4. This comprehensive analysis provides valuable insights into the genetic relationships and evolutionary history of BCoV strains.
3. Results
3.1. Frequency of BCoV in Yunnan
In our study, we tested 285 sera from seven cities or prefectures in Yunnan Province for BCoV antibodies. The overall serum antibody positive rate was found to be 21.75% (65/285). When comparing serum antibody positive rates of BCoV in diarrheic and healthy cattle, we observed a higher rate in diarrheic animals at 25.56% (46/180) than in healthy cattle, which had a rate of 15.24% (16/105; Supporting Information 1: Table S1). Among the locations tested, Baoshan City presented the highest serum antibody positive rate of BCoV at 93.33% (28/30), followed by Kunming City with 53.85% (14/26) and Chuxiong Prefecture with 42.55% (20/47). No BCoV serum antibodies were detected in serum samples from Qujing City, Zhaotong City, Honghe Prefecture, and Lincang City. An analysis of different cattle breeds revealed that shorthorn cattle had the highest BCoV seroprevalence at 53.85% (14/26), followed by Dianzhong yellow cattle at 42.55% (20/47), and Hereford cattle at 20.29% (28/138). Notably, no BCoV serum antibodies were detected in serum samples from Angus and Simmental cattle (Figure 1A).

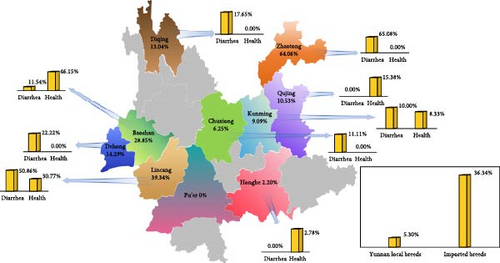
The study analyzed 616 fecal samples from 11 cities or prefectures in Yunnan for BCoV pathogens, revealing an overall positive rate of 28.73% (177/616). More specifically, the total positive rate of BCoV was 37.46% (112/229) in diarrhea cattle and 20.50% (65/317) in healthy cattle (Supporting Information 2: Table S2). Zhaotong City had the highest positivity rate at 64.06% (41/64), followed by Lincang City at 39.34% (107/272), Baoshan City at 28.85% (15/52), Dehong Prefecture at 14.29% (4/28), Diqing Prefecture at 13.04% (3/23), Qujing City at 10.53% (2/19), Kunming City at 9.09% (2/22), Chuxiong Prefecture at 6.25% (1/16), and Honghe Prefecture at 2.20% (2/91). Notably, no BCoV was detected in the fecal samples from Pu’er City. When comparing local and imported breeds, we found a lower BCoV positivity rate of 5.30% (8/151) among the 151 fecal samples obtained from ten Yunnan local breeds (Red River yellow cattle, Diandongnan buffalo, Jiangcheng yellow cattle, Wenshan cattle, Murrah buffalo, Yunnan humped cattle, Mithun cattle, Dehong buffalo, Diqing yellow cattle, and Dianzhong cattle). In contrast, among the 465 fecal samples obtained from six imported breeds (Shorthorn cattle, Angus cattle, Hereford cattle, Holstein cattle, Jersey cattle, and Simmental cattle), the BCoV positivity rate was higher at 36.34% (169/465; Figure 1B).
3.2. Amplification of BCoV Complete Genomic Sequence
Three BCoV-positive samples, identified and stored previously by our laboratory and named BCoV/YN1LC/2023, BCoV/YNZT/2023, and BCoV/YNLP/2023, underwent segmented amplification using 16 pairs of primers designed in this study (Table 1). The study successfully obtained three BCoV epidemic strains BCoV/YN1LC/2023 (GenBank accession numbers: OR088593), BCoV/YNZT/2023 (OR454204), and BCoV/YNLP/2023 (OR753439). BCoV/YN1LC/2023 was identified in the feces of Holstein dairy cows with diarrhea in Lincang City. BCoV/YNZT/2023 was identified in the feces of Hereford cattle with diarrhea in Zhaotong City. BCoV/YNLP/2023 was identified in the feces of Simmental cattle with health in Qujing City. The total lengths of BCoV/YN1LC/2023, BCoV/YNZT/2023, and BCoV/YNLP/2023 are 31,005, 30,992, and 31,006 bp, respectively.
3.3. Sequence Analysis of BCoV Epidemic Strain Identified in Yunnan
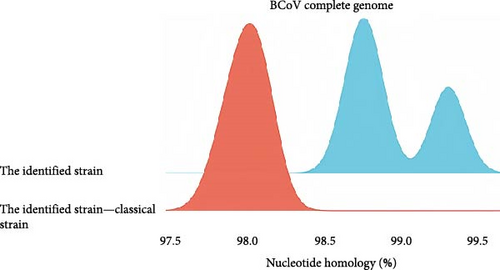
The nucleotide homology analysis of the three BCoV complete genomes obtained in this study is depicted in Figure 2A. The nucleotide homology between BCoV/YN1LC/2023, BCoV/YNZT/2023, and BCoV/YNLP/2023 ranged from 98.7% to 99.3%. Among them, the highest nucleotide homology was found between BCoV/YN1LC/2023 and BCoV/YNZT/2023 at 99.3%. The nucleotide homology between the identified strains and the three classical strains Mebus (U00735.2), Kakegawa (AB354579.1), and Quebuc (AF220295.1) ranged from 97.8% to 98.6%. This high level of homology suggests a close relationship among these strains, reflecting their common ancestry and potentially similar epidemiological characteristics.
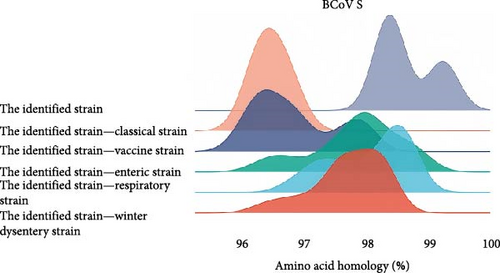
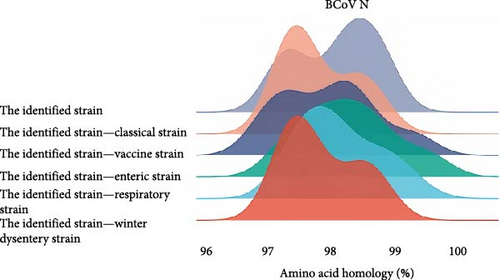
For homology comparison, we selected classical strains, vaccine strains, and strains derived from calf diarrhea samples, winter dysentery samples, and respiratory samples from the NCBI database. Detailed information of reference strains is shown in the Supporting Information 3: Table S3. The results showed that the amino acid homology of the S protein of the BCoV/YN1LC/2023, BCoV/YNZT/2023, and BCoV/YNLP/2023 was between 98.3% and 99.2%. The highest homology (99.2%) was observed between BCoV/YN1LC/2023 and BCoV/YNZT/2023. The amino acid homology between the identified strain and the classical strain was between 96.1% and 96.8%. The amino acid homology between the identified strain and the vaccine strain ranged from 96.0% to 98.0%. The amino acid homology between the identified strains and those derived from calf diarrhea samples was between 96.2% and 98.9%. The amino acid homology between the identified strains and those derived from respiratory tract symptom samples was between 96.8% and 98.7%. The amino acid homology between the identified strains and those derived from winter dysentery samples ranged from 96.1% to 98.4% (Figure 2B). These results suggest considerable conservation amongst these strains but also highlight some variability, which may have implications for disease manifestation and vaccine efficacy.
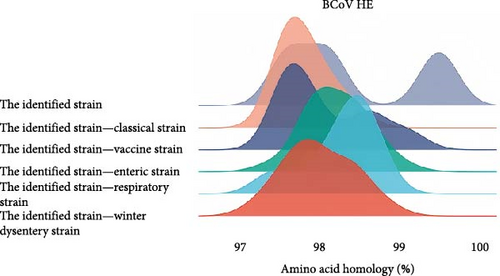
The amino acid homology of HE strains identified by our laboratory in Southwest China ranged from 97.6% to 99.5%, among which BCoV/YN1LC/2023 and BCoV/YNZT/2023 had the highest homology of 99.5%. The amino acid homology between the identified strains and the classical strains was between 97.6% and 98.4%. The amino acid homology between the identified strains and the vaccine strain ranged from 97.6% to 99.1%. In comparisons with disease-specific strains, the identified strains exhibited the following homologies: For strains derived from the calf diarrhea samples, the homology was between 97.4% and 99.1%; for strains derived from the respiratory symptom samples, the homology ranged from 97.4% to 98.8%; last, for strains derived from the winter dysentery symptom samples, the homology was between 97.2% and 98.8%. (Figure 2C). These high levels of homology indicate significant similarity among these strains at the amino acid level. This could have implications for cross-protection among different BCoV strains and potentially influence the design and efficacy of vaccines.
The amino acid homology analysis of the N protein of BCoV strains is depicted in Figure 2D. The amino acid homology between the identified strains and the classical strains was between 97.3% and 98.7%. The amino acid homology between the identified strain and the vaccine strain ranged from 97.1% to 99.3%. In the case of strains derived from calf diarrhea samples, the homology ranged from 97.1% to 99.6%. The amino acid homology between the identified strains and those derived from respiratory tract symptom samples was between 97.3% and 99.3%. Last, for strains derived from winter dysentery symptom samples, the homology was between 97.3% and 98.7%. These high levels of amino acid homology among different BCoV strains suggest a significant degree of conservation in the N protein, potentially indicating its importance in the virus’s life cycle and pathogenicity.
The amino acid sequences of the key structural proteins (S, N, and HE) of the three BCoV epidemic strains identified in this study were analyzed against the representative strains extracted from GenBank, focusing on sites of amino acid difference (Figure 3). Detailed information of reference strains is shown in the Supporting Information 4: Table S4. A comparison of S protein amino acid sequences showed that the three identified strains shared several mutations sites: M11/T, K115/D, N146/I, D148/G, L154/F, N499/S, and N509/H, when compared to the classical strains. BCoV/YN1LC/2023 and BCoV/YNZT/2023 exhibited Chinese strain-specific mutation patterns at positions 121 and 256, with mutations E121/V and M256/L, respectively. Remarkably, BCoV/YNLP/2023 displayed a unique mutation at position 501 identified as S501/F (Figure 3A). These findings reveal specific mutation patterns in the S protein of these strains, which may affect their interaction with host cells and possibly influence their virulence, pathogenicity, or transmissibility.
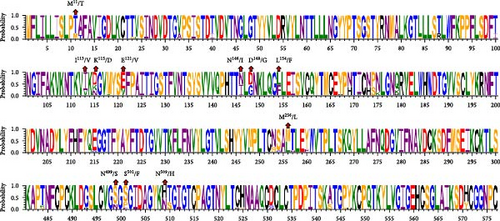
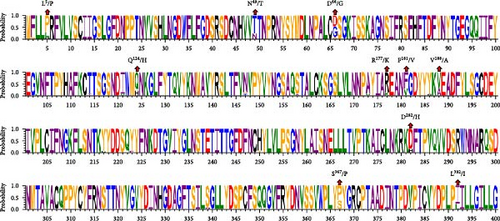
The comparison of the HE protein amino acid sequences revealed that the three identified strains shared several mutation sites: L5/P, N49/T, and L392/I, in comparison to the classical strains. BCoV/YN1LC/2023 and BCoV/YNZT/2023 share mutations at positions 181 and 188, specifically F181/V and V188/A. They also exhibited unique mutations at positions 177 and 282, namely, R177/K and D282/H, when compared to the reference strains. Mutations Q124/H and S367/P were shared between BCoV/YNZT/2023 and BCoV/YNLP/2023. Moreover, BCoV/YNLP/2023 had a mutation site in common with the Korean strain, which was D66/G (Figure 3B). This analysis reveals specific mutation patterns in the HE protein of these strains, potentially affecting their functionality or interaction with host cells.
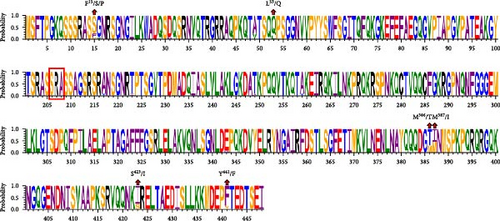
The comparison of the N protein amino acid sequences indicated that the three identified strains shared mutation sites at L53/Q, M386/T, M387/I, S423/I, and Y441/F when compared to the classical strains. BCoV/YN1LC/2023 and BCoV/YNLP/2023 shared a mutation at position F15/S. However, BCoV/YNZT/2023 exhibited a distinct mutation at position F15/P. Interestingly, a deletion found in BCoV/YNLP/2023, spanning from locus 206 to locus 208, which is SRA, compared to the classical strains (Figure 3C). These findings highlight specific mutation patterns in the N protein of these strains, potentially impacting their functionality or interaction with host cells.
3.4. Phylogenetic Analysis of BCoV Strains
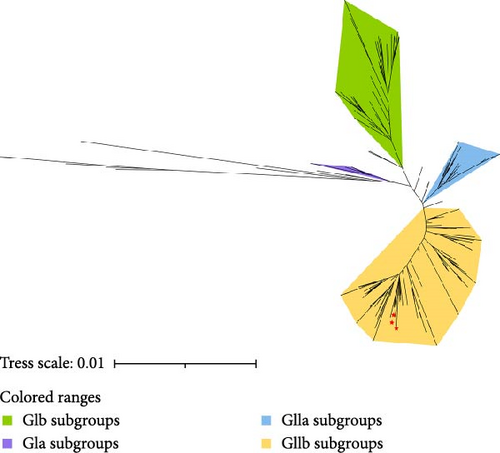
For phylogenetic analysis, the entire genomes of various BCoV strains from different geographical regions within China and across the world were retrieved from the NCBI nucleotide database to serve as reference sequences (Figure 4). The phylogenetic analysis based on whole-genome sequences revealed that the three BCoV strains identified in this study and the 197 reference sequences could be grouped into four clades: GIa subgroups (4/200, 2.00%), GIb subgroups (31/200, 15.50%), GIIb subgroups (151/200, 75.50%), and other species subgroups (14/200, 7.00%). The circulating strains identified by our laboratory in Southwest China were classified under the GIIb subgroups. It was found that the strains BCoV/YN1LC/2023 and BCoV/YNLP/2023 exhibited high homology similarity. BCoV/YNLP/2023 formed a separate small clade, closely associated with HMsz2207 and NMG11.
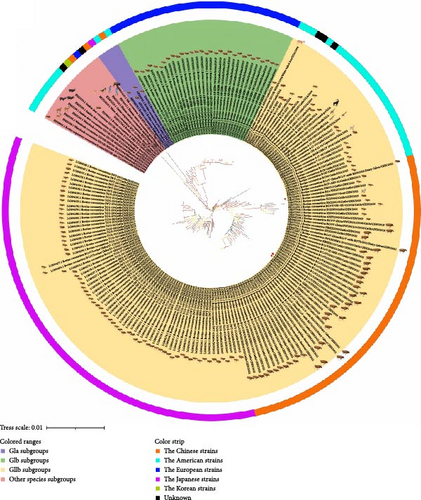
A phylogenetic tree was constructed using the ML method based on the complete sequence of the S gene (Figure 5). The analysis revealed that the BCoV S gene could be categorized into four main branches: classical strains congregated in one branch (GIa type), European strains formed a distinct clade (GIb type), Korean strains clustered in a separate clade (type GIIa), and American and Chinese strains were closely related in a single clade (type GIIb). The circulating strains identified by our laboratory were divided into a small branch within the GIIb subgroup. In addition to these, several smaller branches were scattered beyond the four principal branches.
3.5. Recombination Analysis of BCoV Strains
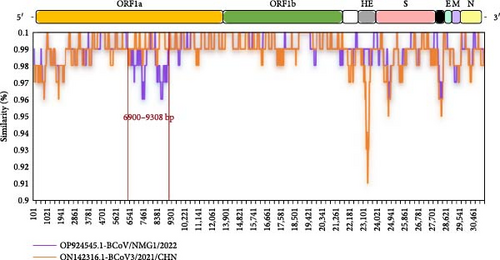
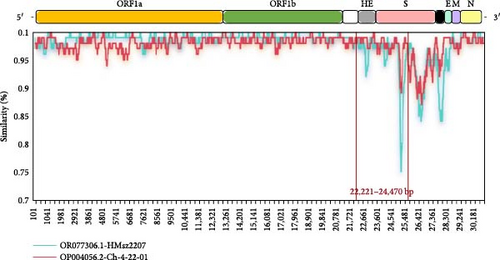
Recombination analysis of the complete genome sequences of BCoV/YN1LC/2023, BCoV/YNZT/2023, and BCoV/YNLP/2023 strains was performed using seven different prediction methods in RDP4 (RDP, GENECONV, BootScan, Maxchi, Chimaera, SiScan, and 3seq). The potential recombination events were then confirmed using SimPlot software. The results showed that only BCoV/YNLP/2023 was found to have genetic recombination. A possible recombination event in BCoV/YNLP/2023 was detected in two fragments by all seven different assays in RDP4 (Table 2), and this finding was validated by SimPlot. BCoV/YNLP/2023 underwent a likely recombination event between the breakpoint positions of 6900–9308 bp (located in the ORF1a region). Here, the Chinese Inner Mongolian strain BCoV/NMG1/2022 (OP924545.1) acted as the major parent and the Chinese Heilongjiang strain BCoV3/2021/CHN (ON142316.1) as the minor parent (Figure 6A). A potential recombination event occurred between the breakpoint positions of 22,221–24,470 bp (located in the HE and S High variation region). In this instance, the Chinese Jiangsu strain HMsz2207 (OR077306.1) served as the major parent, with the American strain CH-4-22-01 (OP004056.2) acting as the secondary parent (Figure 6B).
| Strain | Detection methods | Recombination signal yes/no | p Value |
|---|---|---|---|
|
RDP | + | 6.675 × 10−09 |
| GENECONV | + | 2.394 × 10−03 | |
| Bootscan | − | — | |
| MaxChi | + | 2.081 × 10−04 | |
| Chimaera | + | 4.000 × 10−03 | |
| SiSscan | − | — | |
| 3Seq | + | 3.132 × 10−06 | |
|
RDP | + | 1.507 × 10−08 |
| GENECONV | + | 4.590 × 10−03 | |
| Bootscan | + | 5.406 × 10−03 | |
| MaxChi | + | 1.223 × 10−04 | |
| Chimaera | + | 6.305 × 10−05 | |
| SiSscan | + | 1.969 × 10−03 | |
| 3Seq | + | 4.425 × 10−07 | |
3.6. Selection Pressure Analysis of the BCoV S Gene
Selection pressure analysis was performed on BCoV S gene sequences, including the three Yunnan epidemic strains identified in this study. The results demonstrated the occurrence of positive selection at sites recognized by at least two algorithms. A total of 10 sites in the BCoV S gene were found to be under positive selection (Table 3). Specific sites under positive selection were located in different regions of the BCoV S protein: sites 113 and 174 were located inside the S1 N-terminal domain (S1-NTD); sites 499, 501, 509, and 510 were situated inside the S1 C-terminal domain (S1-CTD); sites 1237, 1296, and 1362 resided inside S2 subunit. These findings highlight the evolutionary pressures acting on specific regions of the BCoV S gene, potentially influencing the virus’s binding affinity, transmissibility, or immune evasion capabilities.
| Site | FUBAR (p Value) | MEME (p Value) | FEL (p Value) | SLAC (p Value) |
|---|---|---|---|---|
| 12 | ≤ 0.001 | 0.02 | 0.044 | 0.039 |
| 113 | 0.002 | 0.03 | 0.043 | 0.060 |
| 174 | 0.016 | 0.06 | 0.094 | 0.058 |
| 499 | 0.001 | 0.03 | 0.042 | 0.051 |
| 501 | ≤ 0.001 | 0.01 | 0.006 | 0.001 |
| 509 | ≤ 0.001 | 0.01 | 0.008 | 0.007 |
| 510 | 0.008 | — | 0.091 | — |
| 1237 | 0.009 | ≤ 0.001 | 0.072 | 0.061 |
| 1296 | 0.056 | 0.04 | 0.034 | — |
| 1362 | ≤ 0.001 | 0.01 | 0.006 | — |
3.7. Estimation of BCoV Differentiation Time and Evolutionary Rate Based on the S Gene
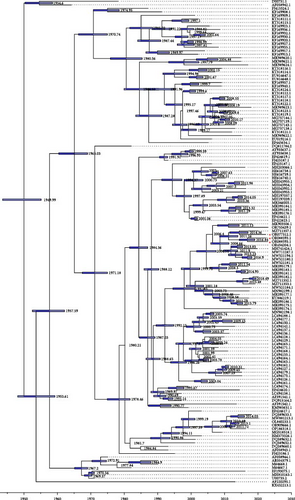
The evolutionary rate (locus/per year) and tMRCA of BCoV were estimated based on S-gene sequences. Our findings demonstrate that the evolutionary rate of BCoV was estimated at 5.055 × 10−4 substitutions per locus per year, with a 95% confidence interval ranging from 4.4448 × 10−4 to 5.6832 × 10−4. Furthermore, the tMRCA for BCoV was estimated to be around 1949.99 years, with a 95% confidence interval spanning from 1944.2348 to 1955.8568. The MCC tree analysis revealed that BCoV/YNZT/2023 and BCoV/YN1LC/2023 were placed on the same branch, suggesting they likely diverged from a common ancestor strain around 2016. On the other hand, BCoV/YNLP/2023 clustered on the same branch as the Hebei, China strain BCoV/CH/HB-BD/2019. This suggests that they may have diverged from the same strain around 2011 (Figure 7). These estimations provide valuable insights into the evolutionary dynamics of BCoV, including its evolutionary rate, divergence times, and potential ancestral relationships among different strains.
4. Discussion
In the study, the total serum antibody positive rate of BCoV detected in Yunnan was higher than those observed in Shanxi, Jilin, and Inner Mongolia in China, but lower than in Ghana, Belgium, and Norway [14, 26–30]. The total positivity rate for BCoV was 28.73% (177/616) in Yunnan, which exceeded rates recorded in Thailand, India, and Vietnam, yet lower than in Canada and Sweden. These findings suggest that BCoV is prevalent in China and globally, with some regional and seasonal variations [31–35]. It was noted that the prevalence of BCoV varied among different cattle breeds. A study [36] detected BCoV in cattle populations in Uruguay in 2019, with a total detection rate of 7.8%. The Angus and Hereford cattle sampled in our study were originally imported from Uruguay. In the study, The Hereford cattle were tested positive for BCoV. This raises the possibility that BCoV may have been introduced or transported from Uruguay.
Interestingly, BCoV was detected in the feces of healthy cattle, suggesting the potential existence of secluded BCoV infection in the Yunnan cattle herd. The persistence of BCoV in the farming environment could be due to diarrheic cattle continuing to shed the virus, thereby, maintaining the farm’s contamination over prolonged periods. Additionally, these diarrheic cattle could potentially act as infection sources, spreading BCoV to nearby healthy cattle. These findings underscore the need for Yunnan to strengthen its monitoring, prevention, and control measures against BCoV and to strictly adhere to the relevant guidelines for introducing and quarantining livestock to prevent further spread of BCoV. Our analysis revealed that BCoV/YN1LC/2023 is genetically closer to BCoV/YNZT/2023 and somewhat more distant from BCoV/YNLP/2023, indicating some degree of genetic variation among these strains. Furthermore, the S and HE proteins of the strains identified in our study showed greater identity to strains derived from calf diarrhea and respiratory samples than to classical and vaccine strains. These findings contribute valuable data to the global scientific community’s understanding of BCoV’s genetic makeup and variability, potentially informing future research and vaccine development efforts.
Comparison of key BCoV proteins revealed that the three identified strains shared seven identical amino acid site mutations in the S protein. Notably, the identified strains had mutations at the N146/I and D148/G sites, similar to mutations found in American strains during the period of 1996–1998 [37]. This suggests that the BCoV strains identified in Yunnan may have followed a similar mutation and evolutionary trajectory as seen in the 1990s. The BCoV S protein has been observed to undergo mutations N146/I and D148/G over time, leading to structural changes [37]. The mutations at amino acid sites 146 and 148 in the S protein, situated near the sialic acid binding site within the NTD region, could impact the binding to the NTD. These variations might differ between respiratory and intestinal strains [38]. The identified strains also showed an N509/H mutation, which is a deviation from the previously reported N509/T [38]. Given that amino acid 509 is located in the receptor-binding domain of the S protein, a site under positive selection pressure, the N509/H mutation may play a significant role in BCoV transmission and alterations in tissue tropism. BCoV/YN1LC/2023 and BCoV/YNZT/2023 exhibit a Chinese strain-specific mutation E121/V in the S1 receptor-binding region. This mutation could potentially alter the receptor-binding capacity and host receptor specificity [39, 40]. Meanwhile, BCoV/YNLP/2023 exhibited a unique mutation, S501/F, aligning with the HLJ/QQHR-6/2020 and HLJ/QQHR-7/2020 strains identified in China in 2020 [41]. The S501/F mutation is located in the putative receptor-binding domain (S1-CTD) on the external surface of BCoV S protein. This mutation could potentially result in structural changes in the S1 protein. These findings highlight the molecular diversity and evolutionary dynamics of BCoV strains and also underscore the potential impact of key mutations on viral properties such as receptor binding and tissue tropism.
The HE protein of all three BCoV strains identified in the Yunnan epidemic had identical mutations at three amino acid sites: L5/P in the signal peptide, N49/T in the putative esterase domain, and L392/I in the membrane-proximal domain. The L5/P mutation was first reported in a Korea strain from 2002 to 2003 and is a conserved virulence-specific mutation found across all EBCVs [42]. Interestingly, BCoV/YNLP/2023 carries the unique D66/G mutation. This mutation has been specifically reported in all respiratory BCoV (RBCV) strains [42]. As evolutionary processes have taken place, the sequence identity between EBCV and RBCV has become increasingly ambiguous. Both BCoV/YN1LC/2023 and BCoV/YNZT/2023 exhibit an F181/V mutation, mirroring those seen in the Chinese recombinant strains KU886219 and MH810163 [17, 43]. Located in the R2-loop of the HE gene, this F181/V substitution could potentially reduce receptor-binding activity due to amino acid substitutions in this region [17, 43]. These observations underline the significant role played by key mutations in the HE protein in shaping the pathogenicity and host interactions of BCoV strains.
The nucleocapsid (N) protein of all three BCoV strains identified in the Yunnan epidemic had identical mutations at five amino acid sites: L53/Q, M386/T, M387/I, S423/I, and Y441/F. Interestingly, BCoV/YNLP/2023 exhibited an SRA deletion at positions 206–208, mirroring those seen in Japanese strains from 2016 to 2017 [44]. This SRA deletion could potentially impact functions such as the formation of genomic RNA complexes by N proteins or interactions with M proteins. BCoV/YNZT/2023 displayed a unique mutation at site F15/P, which diverges from other strains and contributes to the high variability at this location. However, the implications of this F15/P mutation on the structural and functional properties of BCoV remain unclear and warrant further investigations.
The whole genome-wide evolutionary tree displayed discrepancies in the classification of BCoV strains. This finding suggests that the strains identified in Yunnan have embarked on a distinct evolutionary path compared to other strains, indicating an apparent trend towards independent evolution. Furthermore, the evolutionary tree of the S gene revealed that all Chinese strains, including the three BCoV strains identified in Yunnan, grouped together within the large branch of American-type strains (GIIb subgroups). Specifically, BCoV/YN1LC/2023, BCoV/YNZT/2023, and BCoV/YNLP/2023 clustered within the same small branch. These observations suggest that that the identified BCoV strains could be the predominant strains in Yunnan Province or potentially even in Southwest China, showing geographic clustering. These findings highlight the importance of ongoing surveillance and genetic analysis for understanding the local epidemiology and transmission dynamics of BCoV strains.
CoVs are known to infect a wide range of mammals and birds. Genetic recombination between CoVs from the same or different species contributes to their genetic diversity, thereby, amplifying the risk of disease spread [45]. Studies have uncovered that BCoV is not confined solely to cattle; BCoV-like pathogens have also been detected in humans and other ruminant species [46, 47]. These pathogens are believed to stem from host variation in BCoV, partly attributed to its proficient ability to recombine genomes. In 2023, Bahoussi et al. [48] carried out recombination analyses on available whole-genome sequences and elucidated that BCoV recombination predominantly occured within a single country, albeit at lower frequencies across national borders. A study by Zhu et al. [41], involving an analysis of the S gene sequences of 20 BCoV strains from Heilongjiang Province in Northeastern China, revealed potential recombination between the HLJ/HH-20/2020 and HLJ/HH-10/2020 strains, leading to the emergence of BCV-AKS-01 recombinant strain. Our study found that BCoV/YN1LC/2023 may have undergone two recombination events, with breakpoints between 6900 and 9308 bp (the recombinant parents being the Chinese strains BCoV/NMG1/2022 and BCoV3/2021/CHN) and between 22,221 and 24,470 bp (the recombinant parents being the Chinese Jiangsu strain HMsz2207 and American strain CH-4-22-01). The 22,221–24,470 bp region, where recombination occurred, encompasses the complete HE protein and S protein highly variable region. Recombination within this region could potentially foster the advent of strains with new genotypes, host ranges, and tissue predilections. Our study also identified ten positive selection sites in the S gene of BCoV, an increase compared to the five selection pressure sites reported by Zhu et al. [41] in 2022. This suggests a high rate of adaptive mutations in the S gene. In summary, the information not only sheds light on the limitations of current vaccine control measures in cattle but also provides new insight for devising effective preventive and control strategies against future BCoV outbreaks.
A study by Kin et al. [49] found that HCoV-OC43 is closely related to BCoV and cross-species transmission of BCoV to humans led to the emergence of HCoV-OC43. They estimated the common ancestor of BCoV and HCoV-OC43 to have diverged around 1890 [49]. Vijgen et al. [19]estimated the evolutionary rate of BCoV to be 4.3 × 10−4 substitutions/locus/year and proposed that the common ancestor of BCoV diverged in the 1940s [50]. In our study, we employed a molecular clock approach to investigate the evolution and divergence time of BCoV strains identified in Yunnan. The analysis revealed an evolutionary rate of BCoV calculated at 5.055 × 10−4 substitutions/locus/year. Furthermore, the estimated tMRCA of BCoV was approximately 1949.99 years, which aligns with the findings by Vijgen et al. [19]. Our study indicates a slight increase in the evolutionary rate of BCoV, suggesting that BCoV is undergoing continuous evolution. The MCC tree analysis indicated that the Yunnan epidemic strains BCoV/YNZT/2023 and BCoV/YN1LC/2023 may have diverged from the same strain in 2016. Additionally, BCoV/YNLP/2023 and the Hebei, China strain BCoV/CH/HB-BD/2019 may have diverged from the same strain in 2011. These findings underscore the risk of cross-regional transmission of BCoV in Yunnan Province.
5. Conclusion
In summary, the study revealed the high prevalence of BCoV in Yunnan and found that the Yunnan BCoV epidemic strains have certain genetic evolution characteristics and geographical aggregation. These findings contribute significantly to our understanding of the genetic evolution of the BCoV strains circulating in Southwestern China.
Ethics Statement
All experiments complied with the Laboratory Animals Guideline of Welfare and Ethics published by the General Administration of Quality Supervision, Inspection, and Quarantine of the People’s Republic of China. The proposals for the animal experiments were approved by the Ethical Committee for Animal Experiments of Yunnan Agricultural University (approval numbers: APYNAU202102003). The respective farm owners provided their consent for both the sample collection and subsequent publication of the data derived from these samples.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, validation: Shanshan Qi and Ying Yang. Resources: Junlong Bi, Gefen Yin, and Yongneng Li. Methodology: Shanshan Qi, Ying Yang, Junwen Deng, and Xinxian Wang. Investigation, data curation: Shanshan Qi, Ying Yang, Junwen Deng, Xinxian Wang, Yongneng Li, Jiaxing Sun, Qian Li, and Shurui Yang. Formal analysis, software, visualization: Shanshan Qi, Ying Yang, and Junwen Deng. Writing–original draft: Shanshan Qi, Ying Yang, and Junwen Deng. Writing–review and editing: Gefen Yin, Junlong Bi, and Yongneng Li. Funding acquisition: Gefen Yin and Junlong Bi. Supervision, project administration: Junlong Bi, Gefen Yin, and Yongneng Li. All authors have read and approved the final manuscript. Shanshan Qi and Ying Yang have equal contribution.
Funding
This work is supported by Yunnan Provincial Innovation Team of Key Technologies for Prevention and Control of Important Livestock and Poultry Diseases (Grant 202405AS350004), Yunnan Provincial Local Universities Joint Special Project-Face-up (Grant 202101BA070001-185), National Natural Science Foundation of China (Grant 32260877), and Yunnan Province High-level Scientific and Technological Talents and Innovative Teams Selection Special Project-Technological Innovation Talents Cultivation Objects (Grant 202105AD160036), and Introduction Special Program—Yunnan Province Key Foreign Expert Project (Grant 202505AO120028).
Acknowledgments
We thank Prof. Jianping Liu for his polishing and constructive comments on the article.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




